PCCU Dosing Handbook 2009 (PDF)
File information
Title: Microsoft Word - Drug book 2009 - final.doc
Author: SHORTTK
This PDF 1.4 document has been generated by PScript5.dll Version 5.2.2 / GPL Ghostscript 8.15, and has been sent on pdf-archive.com on 04/07/2015 at 01:18, from IP address 198.84.x.x.
The current document download page has been viewed 719 times.
File size: 598.32 KB (53 pages).
Privacy: public file




File preview
CHILDREN’S HOSPITAL
LONDON HEALTH SCIENCES CENTRE
Dosing Guidelines for
Drugs used in the
Paediatric Critical Care Unit
2009 Edition
Children’s Hospital, London Health Sciences Centre
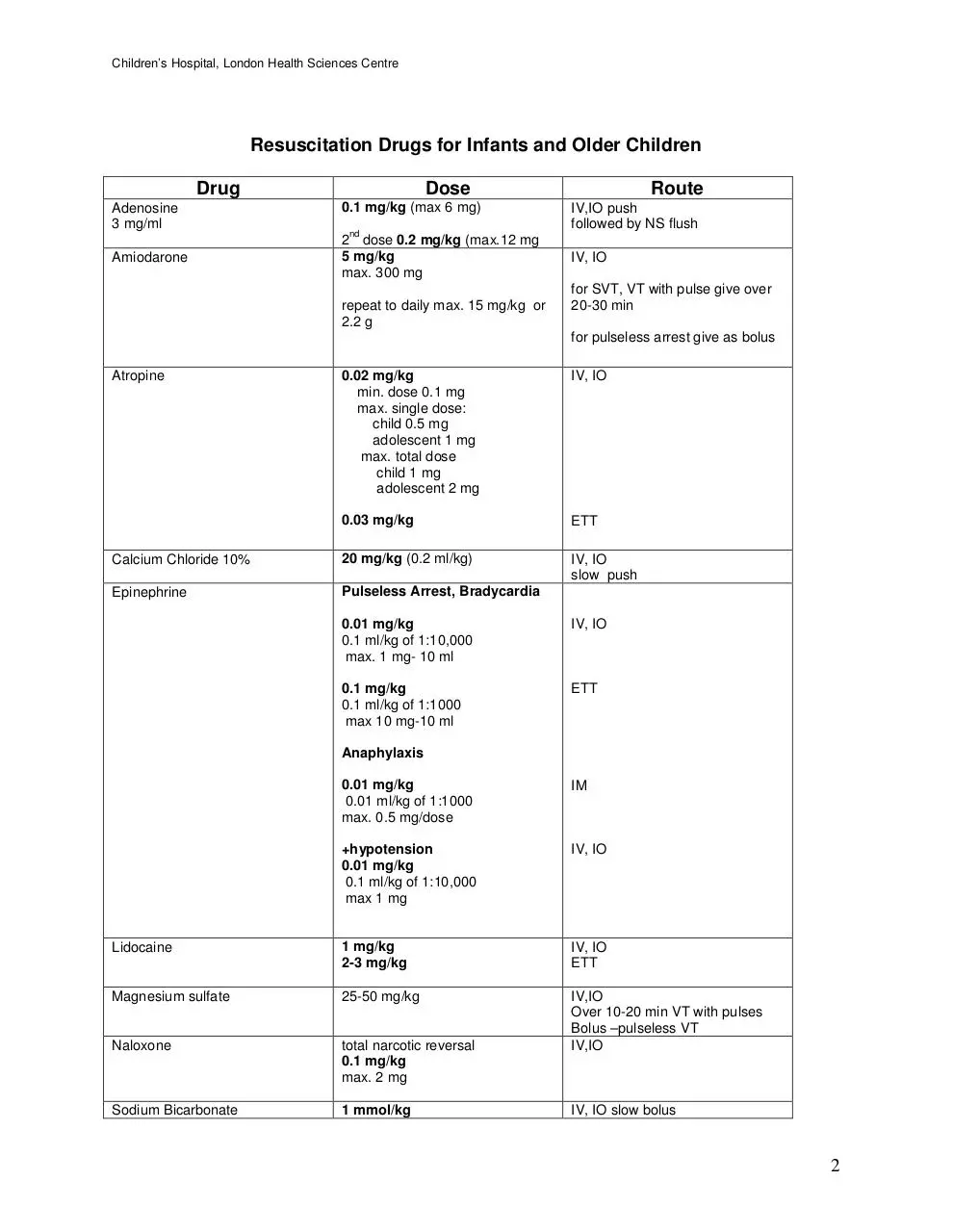
Resuscitation Drugs for Infants and Older Children
Drug
Adenosine
3 mg/ml
Amiodarone
Dose
0.1 mg/kg (max 6 mg)
nd
2 dose 0.2 mg/kg (max.12 mg
5 mg/kg
max. 300 mg
repeat to daily max. 15 mg/kg or
2.2 g
Route
IV,IO push
followed by NS flush
IV, IO
for SVT, VT with pulse give over
20-30 min
for pulseless arrest give as bolus
0.02 mg/kg
min. dose 0.1 mg
max. single dose:
child 0.5 mg
adolescent 1 mg
max. total dose
child 1 mg
adolescent 2 mg
IV, IO
0.03 mg/kg
ETT
Calcium Chloride 10%
20 mg/kg (0.2 ml/kg)
IV, IO
slow push
Epinephrine
Pulseless Arrest, Bradycardia
Atropine
0.01 mg/kg
0.1 ml/kg of 1:10,000
max. 1 mg- 10 ml
IV, IO
0.1 mg/kg
0.1 ml/kg of 1:1000
max 10 mg-10 ml
ETT
Anaphylaxis
0.01 mg/kg
0.01 ml/kg of 1:1000
max. 0.5 mg/dose
IM
+hypotension
0.01 mg/kg
0.1 ml/kg of 1:10,000
max 1 mg
IV, IO
Lidocaine
1 mg/kg
2-3 mg/kg
IV, IO
ETT
Magnesium sulfate
25-50 mg/kg
Naloxone
total narcotic reversal
0.1 mg/kg
max. 2 mg
IV,IO
Over 10-20 min VT with pulses
Bolus –pulseless VT
IV,IO
Sodium Bicarbonate
1 mmol/kg
IV, IO slow bolus
2
Children’s Hospital, London Health Sciences Centre
DOSING GUIDELINES FOR DRUGS USED IN THE
PAEDIATRIC CRITICAL CARE UNIT
Reprinted with permission
INTENDED FOR USE IN THE PAEDIATRIC CRITICAL CARE UNIT (PCCU),
CHILDREN’S HOSPITAL, LONDON HEALTH SCIENCES CENTRE,
LONDON, ONTARIO.
Intended only to serve as a guide; current literature sources and Paediatric
Pharmacists should be consulted for further information.
NOTE:
When treating patients with liver and/or kidney impairment consult
further references for possible dosage adjustments.
Legend
A
Adult
N
Neonate-doses apply to newborn infants until
post-conceptional age >38 weeks and post-natal
age of >4 weeks
C
Child
I
Infant
ETT
Endotracheally
Inh
Inhalation
IM
Intramuscular
IO
Intraosseus
IV
Intravenous
PNA
Post natal age
po
Oral
pr
Rectal
Renal dose
adjust
sc
Dose requires adjustment in renal insufficiency
consult other references
subcutaneous
3
Children’s Hospital, London Health Sciences Centre
Intended for use in the Paediatric Critical Care Unit (PCCU)
Children’s Hospital, London Health Sciences Centre, London, Ontario.
Compiled from a review of the literature and reviewed by L. Burril, S. Campbell,
M. Edwards RD., Dr. D. Fraser, Dr. A. Kornecki, Dr. D. Matsui, Dr. G. Morrison,
Dr. A. Price, Dr. M.J. Rieder, Dr. M. Salvadori, Dr. R. Singh,
Prepared by:
The Department of Pharmacy Services
Intended only to serve as a guide; current literature sources and Paediatric
Pharmacists should be consulted for further information,
NOTE: when treating patients with liver and/or kidney impairment consult
further references for possible dosage adjustments
Copyright September 1986 Revised: 02/87; 06/87; 06/88; 06/89; 06/90; 06/91;
05/92; 06/93; 08/94; 07/96; 05/99; 08/02; 06/03; 07/08
4
Children’s Hospital, London Health Sciences Centre
Children’s Hospital, London Health Sciences Centre, 2008
Table of Contents SECTION PAGE
Drug List.......................................................................
Adrenal Coticosteriod Comparison Chart.....................
Anticoagulation Nomograms ........................................
Continuous Infusion Guide...........................................
Drug Infusions ..............................................................
Fluid Requirements ......................................................
TPN Recommendations/Formulas ...............................
Enternal Feedings ........................................................
Feeding Protocol ..........................................................
Chest Tube/ETT sizes..................................................
Umbilical Catheters ......................................................
Notes............................................................................
6
34
35
36
37
38
39
41
44
46
47
48
5
Children’s Hospital, London Health Sciences Centre
DRUG
AGE/COMMENTS
po/pr q4-6h
5mg/kg/dose
5 mg/kg/dose
5 mg/kg/dose
(↑ by 25 mg/kg/day to
max. 100 mg/kg/day)
po, IV q24h
po, IV q8-12h
po, IV q6h
60-100 mg/kg/day
po div q6h
80-100 mg/kg/day
po div q6h
Maintenance
3-5 mg/kg/day
po q 24h
Antiplatelet
3-5 mg/kg/day
(max. 325 mg/day)
po q24h
Neonatal HSV
60 mg/kg/day
IV div q8h
I, C
HSV encephalitis
1 mo-12 yr
> 12y
60 mg/kg/day
30 mg/kg/day
IV div q8h
IV div q8h
N, I, C
acetazolamide
I, C
Diuretic
Urinary alkalinization
Decrease CSF
production
renal dose adjust
acetylsalicylic acid (ASA)
acyclovir
dose IBW
renal dose adjust
ROUTE
FREQUENCY
10-15 mg/kg/dose
Max. dose N 60
mg/kg/day
max. dose I,C 75
mg/kg/day or 4
gm/day whichever is
less)
acetaminophen
IV-SAP product
DOSE
I, C
JRA, pericarditis,
Rheumatic fever
Kawasaki disease
Acute
N
HSV –
immunocompromised
Treatment
15-30 mg/kg/day
80 mg/kg/day
(max. 1000 mg/day)
IV div q8h
po div 3-5 x /day
600-1000 mg/day
(max. 80 mg/kg/day)
po div 3-5 x /day
IV div q8h
IV div q8h
Immunocompetent
30 mg/kg/day
2
1500 mg/m /day
or
30 mg/kg/day
80 mg/kg/day
Zoster- immunocompetent
≥ 12 years
4000 mg/day
po div 5 x/day
Prophylaxis
Varicella-Zoster
immunocompromised
<1 year
≥ 1 year
CMV prophylaxisimmunocompromised
2
1500 mg/m /day
800-3200 mg/day
(max 80 mg/kg/day)
po div q6h
IV div q8h
po div q6-24h
6
Children’s Hospital, London Health Sciences Centre
DRUG
adenosine
AGE/COMMENTS
ROUTE
FREQUENCY
DOSE
N
0.05 mg/kg
increase by
increments 0.05
mg/kg/dose to max
0.25 mg/kg/dose
IV, IO
I, C
0.1 mg/kg/dose (max.
6mg)
followed by 0.2 mg/kg
(max. 12 mg) in 1-2
min prn
IV, IO
Aldactazide
(hydrochlorothiazide/spironolactone)
I, C
2-4 mg/kg/day
po div q6-12h
allopurinol
C≤ 10
10 mg/kg/day
2
or 200-300 mg/m /day
(max 800 mg/day)
po div q8-12h
po div q6-12h
C>10, A
600-800 mg/day
po div q8-12h
I, C
0.05-1.0 mcg/kg/min
IV
renal dose adjust
alprostadil
may reduce dose to
0.025 mcg/kg/min by
titrating to the patency
of patent ductus
arteriosus
alteplase
Blocked catheter
≤ 10 kg
≥ 10 kg
amiodarone
Leave in lumen 2-4
hours then remove
1mg/ml (amount
required to fill volume
of lumen)
(max. 2 ml)
PALS dose
Pulseless VT/VF
Perfusing tachycardia
PSVT
amlodipine
0.5 mg diluted in NS
to volume required to
fill lumen
5 mg/kg
5 mg/kg
(may repeat to max.
15 mg/kg or 300 mg)
IV,IO rapid
IV over 20-60 min
5mg/kg
followed by 5-10
mcg/kg/min
IV over 60 min
LD:10 mg/kg/day
MD: 5.0 mg/kg/day
po div q12-24h(x 710 days)
po div q24h
0.1 mg/kg/day
0.1-0.3 mg/kg/day
po div q24h
po div q24h
I, C
Initial dose
Maintenance
7
Children’s Hospital, London Health Sciences Centre
DRUG
AGE/COMMENTS
DOSE
ROUTE
FREQUENCY
amoxicillin
N, I
20-30 mg/kg/day
po div q12h
renal dose adjust
I>3 months,C
Acute otitis media
25-50 mg/kg/day
80-90 mg/kg/day
po div q8h
po div q8h
Apslenic prophylaxis
(up to 5 years of age)
20 mg/kg/day
po div q12h
N,I< 3 mo
(4:1 formulation)
30 mg
amoxicillin/kg/day
po div q12h
I>3 mo, C<40kg
(7:1 formulation)
25-45 mg
amoxicillin/kg/day
80-90 mg
amoxicillin/kg/day
po div q12h
amoxicillin clavulanate (Clavulin)
renal dose adjust
limit clavulanate dose 10 mg/kg/day
in children
po div q8-12h
dosing recommendations based
on 4:1 formulation for N, I< 3 mo
7:1 formulation for I>3mo, C
Tablets for C>40kg, A
C>40 kg, A
250-500 mg/dose
(as 500 mg tabs)
po q8h
amphotericin B lipsomal
I, C, A
3 mg/kg/day
IV div q24h
ampicillin
N
PNA < 7 days
<2 kg
meningitis
50 mg/kg/day
100 mg/kg/day
IV div q12h
IV div q12h
>2 kg
meningitis
Group B streptococcus
75 mg/kg/day
150 mg/kg/day
200 mg/kg/day
IV div q8h
IV div q8h
IV div q8h
N
PNA >7 days
<2 kg
meningitis
75 mg/kg/day
150 mg/kg/day
IV div q8h
IV div q8h
> 2 kg
meningitis
Group B streptococcus
100 mg/kg/day
200 mg/kg/day
300 mg/kg/day
IV div q6h
IV div q6h
IV div q6h
I,C
meningitis
100-200 mg/kg/day
200-400 mg/kg/day
(max. 12 g/day)
IV div q6h
IV div q6h
renal dose adjust
8
Children’s Hospital, London Health Sciences Centre
DRUG
atropine
AGE/COMMENTS
ROUTE
FREQUENCY
DOSE
0.02 mg/kg/dose
I, C, A
IV, IO
min dose 0.1 mg
max. single dose:
child 0.5 mg
adolescent 1 mg
max. total dose
child 1 mg
adolescent 2 mg
azithromycin
0.03 mg/kg
ETT
10 mg/kg/day
po div q24h x 5
days
Day 1
10 mg/kg/day
max. 500 mg
po, IV once
Day 2-5
5 mg/kg/day
250 mg
po, IV div q24h
I < 6 mo
Pertussis
C≥ 6 mo
Adolescents ≥ 16 years, A
Day 1
Day 2-5
baclofen
budesonide
max.
500 mg
250 mg
po, IV once
po, IV div q24h
10-15 mg/ day
titrate dose q3days in
increments of 5-15
mg/day
(max. 40 mg/day)
po div q8h
≥ 8 years
titrate as above (max.
60 mg/day)
po div q8h
A
5 mg/dose
may increase by 5
mg/dose q3 days
(max. 80 mg/day)
po q8h
Severe acute asthma
Maintenance
500-1000 mcg/dose
250-500 mcg/dose
Inh q12h
Inh q12h
Severe acute asthma
Maintenance
1000-2000 mcg/dose
500-1000 mcg/dose
Inh q12h
Inh q12h
10 mg caffeine
base/kg
2.5 mg caffeine
base/kg/day
IV, po
po div q6h
C 2-7 years
Initial dose
I,C
A
caffeine
N
Loading dose
Maintenance
calcium carbonate
N
50-150 mg elemental
Ca/kg/day
suspension provides 80 mg
elemental Ca/ml
2mmol elemental Ca/ml
C
45-65 mg elemental
Ca/kg/day
IV, po div q24h
po div q6h
9
Download PCCU Dosing Handbook 2009
PCCU Dosing Handbook 2009.pdf (PDF, 598.32 KB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000285040.