00 2 (PDF)
File information
Title: 00.xps
This PDF 1.6 document has been generated by Adobe Acrobat 11.0.5 / Adobe Acrobat 11.0.5 XPS Conversion Plug-in, and has been sent on pdf-archive.com on 23/11/2015 at 22:11, from IP address 41.129.x.x.
The current document download page has been viewed 808 times.
File size: 139.2 KB (21 pages).
Privacy: public file




File preview
Dilution
&
Concentration
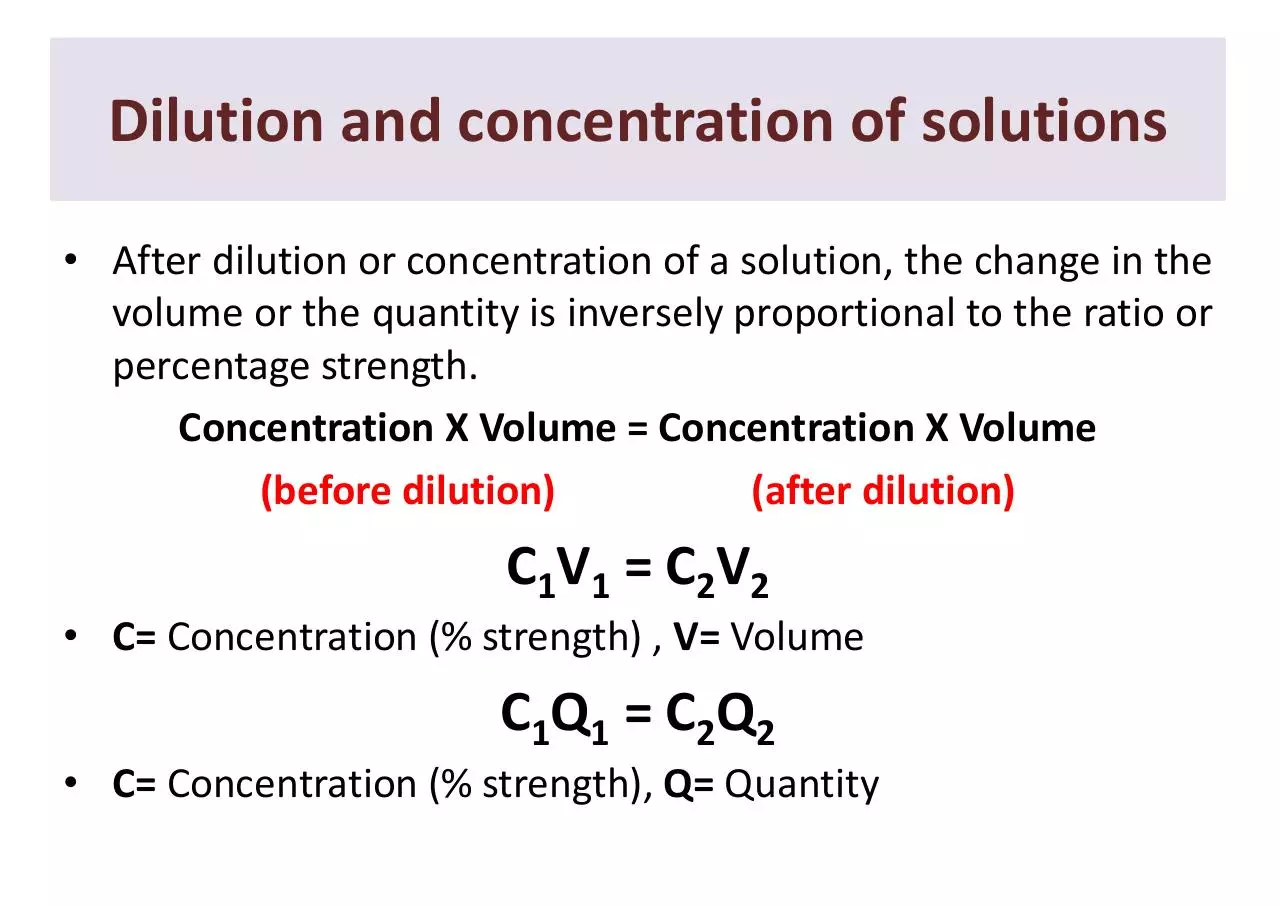
Dilution and concentration of solutions
• After dilution or concentration of a solution, the change in the
volume or the quantity is inversely proportional to the ratio or
percentage strength.
Concentration X Volume = Concentration X Volume
(before dilution)
(after dilution)
C1V1 = C2V2
• C= Concentration (% strength) , V= Volume
C1Q1 = C2Q2
• C= Concentration (% strength), Q= Quantity
Dilution and concentration of solutions
• Ex: If 500 mL of a 15% v/v solution of methyl salicylate in
alcohol is diluted to 1500 mL, what is the percentage
strength v/v?
Solution
C1V1 = C2V2
15 (%) X 500 (ml) = C2 X 1500 (ml)
C2= (15*500)/1500 = 5%
Dilution and concentration of solutions
• Ex: If 50 mL of a 1:20 w/v solution of aluminum acetate is
diluted to 1000 mL, what is the ratio strength w/v?
Solution
1:20= 5%
C1V1 = C2V2
5 (%) X 50 (ml) = C2 X 1000 (ml)
C2= (5*50)/1000 = 0.25% = 1:400
Alligation
• Alligation
is
a
practical
method
of
solving arithmetic problems related to mixtures
of ingredients.
a) Alligation medial: is used to determine the average
percent strength of a mixture of two or more
ingredients of known quantities and concentration.
b) Alligation alternate: is used to determine the
number of parts of two or more components of a
given strength, required to give a mixture of the
desired strength.
Alligation medial
(1) Find the percent strength of ethyl alcohol in a mixture
obtained by mixing 300 ml of 40% , 100 ml of 60% and
100 ml of 70% solutions of ethyl alcohol.
% strength
40
60
70
Volume
Alcohol 1
X
300
= 12,000
Alcohol 2
X
100
= 6,000
Alcohol 3
X
100
= 7,000
500
25,000
% strength of mixture= 25,000/500= 50%
Alligation medial
(2) Find the concentration of NaOH when 2000 ml of 20%,
4000 ml of 10% solutions and 4000 ml water are mixed
together.
% strength
20
10
0
Volume
20% NaOH
X
2000
= 40,000
10% NaOH
X
4000
= 40,000
Water
X
4000
=0
10,000
80,000
Concn of NaOH= 80,000/10,000= 8%
Alligation medial
(3) Find the concentration of Zinc oxide ointment prepared
by mixing 500 g of 50% , 300 g of 40% zinc oxide
ointments and 200 g of Vaseline.
% strength
50
40
0
Amount
50% oint.
X
500
= 25,000
40% oint.
X
300
= 12,000
Vaseline
X
200
=0
1000
37,000
Concn of ZnO oint.= 37,000/1000= 37%
Alligation alternate
(1) In what proportion should 15% boric acid be mixed
with Vaseline to produce 2% boric acid ointment?
% given
Boric acid
Desired concn
15%
Proportional parts required
2
2 parts of boric acid
13
13 parts of Vaseline
2%
Vaseline
0%
15 parts
Boric acid : Vaseline= 2:13
Download 00-2
00-2.pdf (PDF, 139.2 KB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000316943.