VAERS Results Form shu (PDF)
File information
This PDF 1.4 document has been generated by Mozilla/5.0 (Windows NT 6.1; WOW64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/49.0.2623.87 Safari/537.36 / Skia/PDF, and has been sent on pdf-archive.com on 28/03/2016 at 14:10, from IP address 188.25.x.x.
The current document download page has been viewed 677 times.
File size: 546.08 KB (51 pages).
Privacy: public file


File preview
The Vaccine Adverse Event Reporting System (VAERS) Results
Hemolytic uremic syndrome
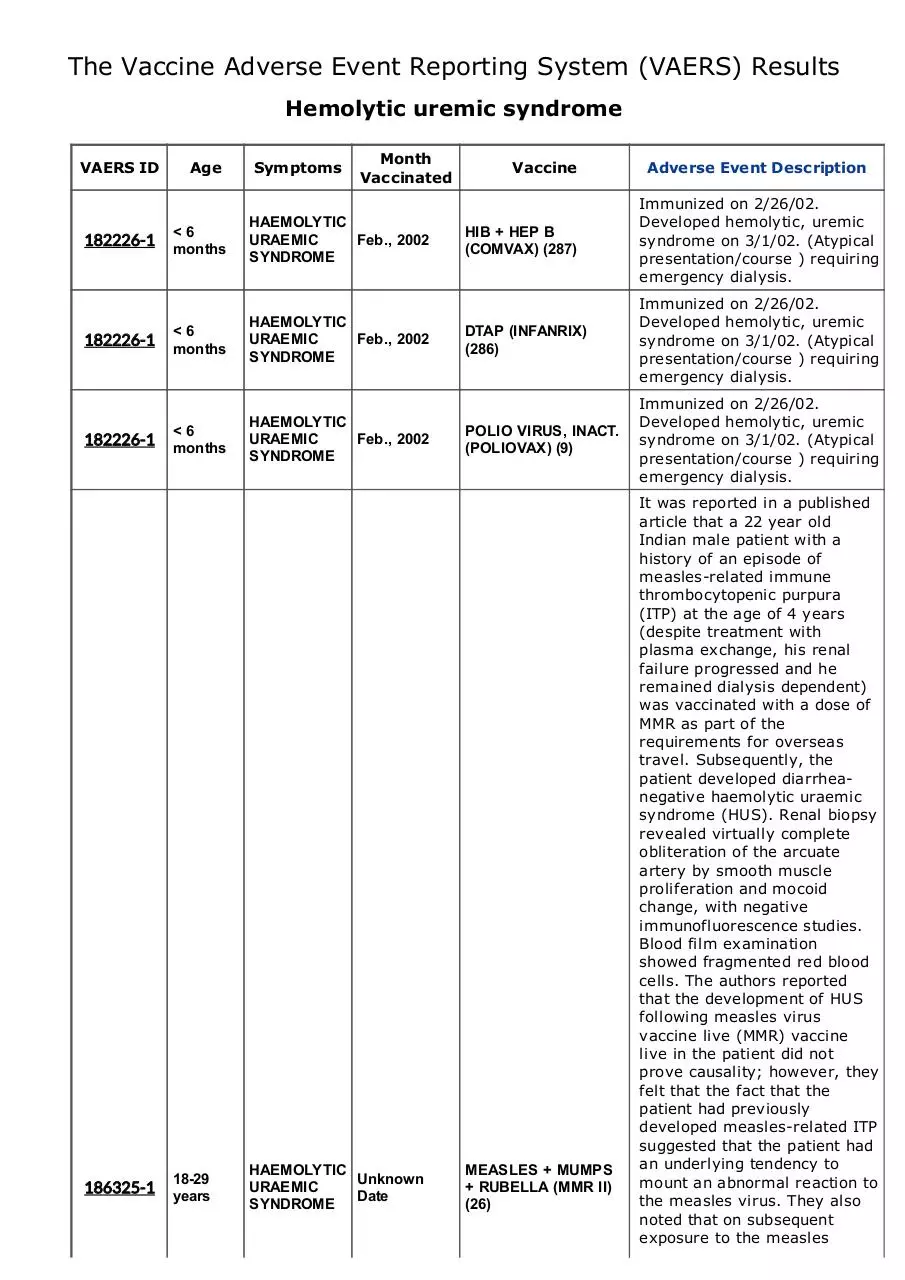
VAERS ID
182226-1
182226-1
182226-1
186325-1
Age
Symptoms
Month
Vaccinated
< 6
months
HAEMOLYTIC
URAEMIC
Feb., 2002
SYNDROME
< 6
months
HAEMOLYTIC
URAEMIC
Feb., 2002
SYNDROME
< 6
months
HAEMOLYTIC
URAEMIC
Feb., 2002
SYNDROME
1829
years
HAEMOLYTIC
Unknown
URAEMIC
Date
SYNDROME
Vaccine
Adverse Event Description
HIB + HEP B
(COMVAX) (287)
Immunized on 2/26/02.
Developed hemolytic, uremic
syndrome on 3/1/02. (Atypical
presentation/course ) requiring
emergency dialysis.
DTAP (INFANRIX)
(286)
Immunized on 2/26/02.
Developed hemolytic, uremic
syndrome on 3/1/02. (Atypical
presentation/course ) requiring
emergency dialysis.
POLIO VIRUS, INACT.
(POLIOVAX) (9)
Immunized on 2/26/02.
Developed hemolytic, uremic
syndrome on 3/1/02. (Atypical
presentation/course ) requiring
emergency dialysis.
MEASLES + MUMPS
+ RUBELLA (MMR II)
(26)
It was reported in a published
article that a 22 year old
Indian male patient with a
history of an episode of
measlesrelated immune
thrombocytopenic purpura
(ITP) at the age of 4 years
(despite treatment with
plasma exchange, his renal
failure progressed and he
remained dialysis dependent)
was vaccinated with a dose of
MMR as part of the
requirements for overseas
travel. Subsequently, the
patient developed diarrhea
negative haemolytic uraemic
syndrome (HUS). Renal biopsy
revealed virtually complete
obliteration of the arcuate
artery by smooth muscle
proliferation and mocoid
change, with negative
immunofluorescence studies.
Blood film examination
showed fragmented red blood
cells. The authors reported
that the development of HUS
following measles virus
vaccine live (MMR) vaccine
live in the patient did not
prove causality; however, they
felt that the fact that the
patient had previously
developed measlesrelated ITP
suggested that the patient had
an underlying tendency to
mount an abnormal reaction to
the measles virus. They also
noted that on subsequent
exposure to the measles
component of the MMR virus
vaccine live, the patient again
developed an abnormal
reaction, which manifested
clinically as HUS. The authors
reported that their case,
together with the previously
reported pediatric case of MMR
virus vaccine live
(WAES0205USA03164)
associated HUS, served to
'flag up' HUS as a potential
complication of MMR virus
vaccine live vaccination,
although does not confirm its
underlying etiology to be MMR
virus vaccine live vaccination.
Upon internal review,
haemolytic uraemic syndrome
was determined to be an other
important medical event/
Additional information has
been requested. This is a
follow up report previously
reported on 03Jun02. This is
an amended report. The
reference WAES number in the
narrative has been changed
from WAES0209USA0314 to
WAES97060729. A copy of the
published article is attached as
further documentation of the
pt's experience. This is a
corrected report as amended.
186326-1
HAEMOLYTIC
Unknown
12 years URAEMIC
Date
SYNDROME
MEASLES + MUMPS
+ RUBELLA (MMR II)
(26)
It was reported in a published
article, that an 18 month old
male pt was vaccinated with a
dose of MMR II vaccine.
Subsequently, the pt presented
with lower limb purpura and a
bloody diarrhea 12 days after
MMR II vaccination. On day 14,
the pt developed acute renal
failure, anemia and
thrombocytopenia. Renal
biopsy revealed thrombotic
microangiopathy, with
negative immune studies.
Polymerase chain reaction
(PCR) for Escherichia coli
0157:H7 on blood and stool
samples was negative. Upon
internal review, haemolytic
uraemic syndrome was
determined to be an other
important medical event.
Additional info has been
requested. This is a follow up
report previously submitted on
03Jun02. It has been
determined that
WAES0205USA03164 is a
duplicate of WAES97060729.
Therefore,WAES0205USA03164
is being deleted from our files
and the report consolidated
into WAES97060729.
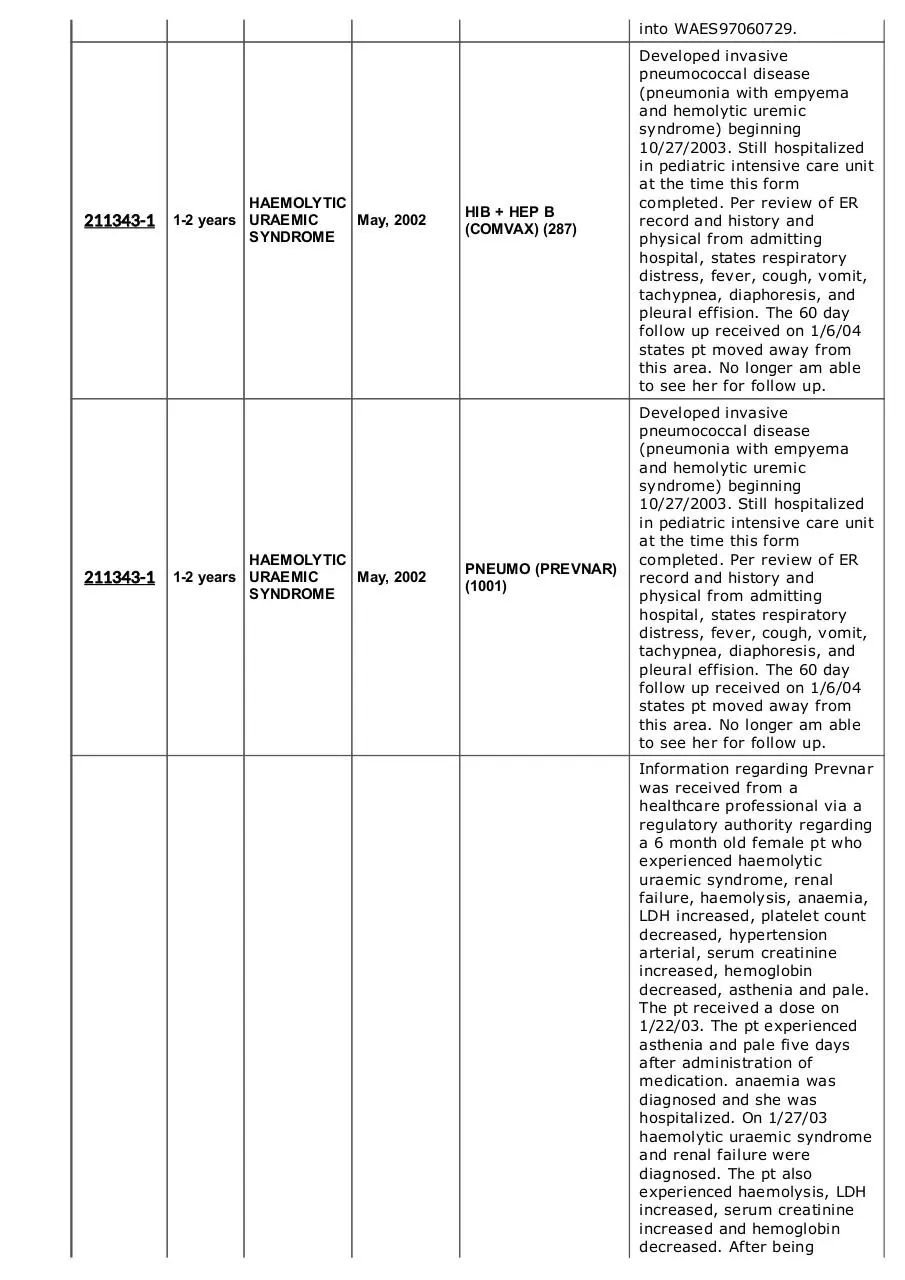
211343-1
211343-1
HAEMOLYTIC
12 years URAEMIC
May, 2002
SYNDROME
HAEMOLYTIC
12 years URAEMIC
May, 2002
SYNDROME
HIB + HEP B
(COMVAX) (287)
Developed invasive
pneumococcal disease
(pneumonia with empyema
and hemolytic uremic
syndrome) beginning
10/27/2003. Still hospitalized
in pediatric intensive care unit
at the time this form
completed. Per review of ER
record and history and
physical from admitting
hospital, states respiratory
distress, fever, cough, vomit,
tachypnea, diaphoresis, and
pleural effision. The 60 day
follow up received on 1/6/04
states pt moved away from
this area. No longer am able
to see her for follow up.
PNEUMO (PREVNAR)
(1001)
Developed invasive
pneumococcal disease
(pneumonia with empyema
and hemolytic uremic
syndrome) beginning
10/27/2003. Still hospitalized
in pediatric intensive care unit
at the time this form
completed. Per review of ER
record and history and
physical from admitting
hospital, states respiratory
distress, fever, cough, vomit,
tachypnea, diaphoresis, and
pleural effision. The 60 day
follow up received on 1/6/04
states pt moved away from
this area. No longer am able
to see her for follow up.
Information regarding Prevnar
was received from a
healthcare professional via a
regulatory authority regarding
a 6 month old female pt who
experienced haemolytic
uraemic syndrome, renal
failure, haemolysis, anaemia,
LDH increased, platelet count
decreased, hypertension
arterial, serum creatinine
increased, hemoglobin
decreased, asthenia and pale.
The pt received a dose on
1/22/03. The pt experienced
asthenia and pale five days
after administration of
medication. anaemia was
diagnosed and she was
hospitalized. On 1/27/03
haemolytic uraemic syndrome
and renal failure were
diagnosed. The pt also
experienced haemolysis, LDH
increased, serum creatinine
increased and hemoglobin
decreased. After being
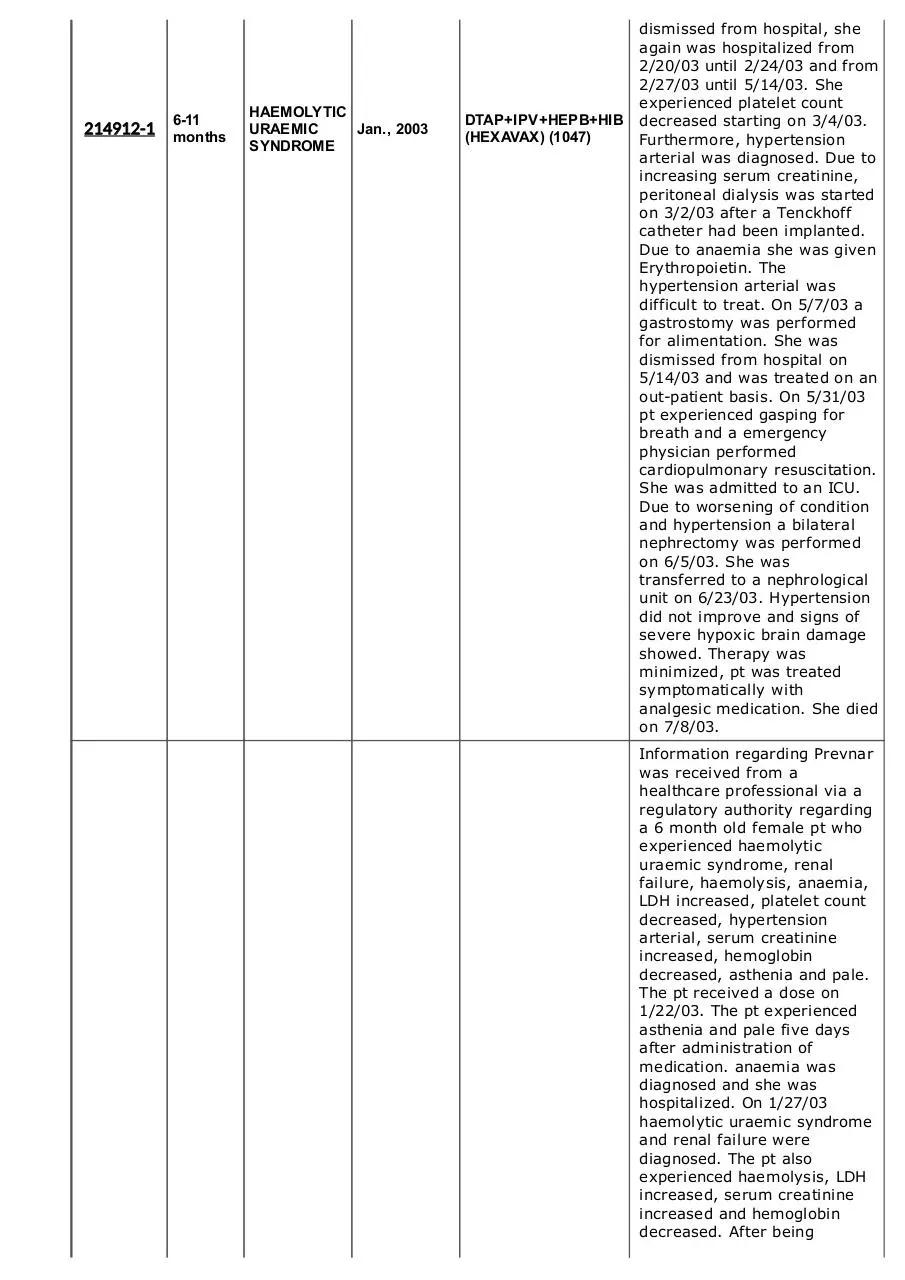
214912-1
611
months
HAEMOLYTIC
URAEMIC
Jan., 2003
SYNDROME
DTAP+IPV+HEPB+HIB
(HEXAVAX) (1047)
dismissed from hospital, she
again was hospitalized from
2/20/03 until 2/24/03 and from
2/27/03 until 5/14/03. She
experienced platelet count
decreased starting on 3/4/03.
Furthermore, hypertension
arterial was diagnosed. Due to
increasing serum creatinine,
peritoneal dialysis was started
on 3/2/03 after a Tenckhoff
catheter had been implanted.
Due to anaemia she was given
Erythropoietin. The
hypertension arterial was
difficult to treat. On 5/7/03 a
gastrostomy was performed
for alimentation. She was
dismissed from hospital on
5/14/03 and was treated on an
outpatient basis. On 5/31/03
pt experienced gasping for
breath and a emergency
physician performed
cardiopulmonary resuscitation.
She was admitted to an ICU.
Due to worsening of condition
and hypertension a bilateral
nephrectomy was performed
on 6/5/03. She was
transferred to a nephrological
unit on 6/23/03. Hypertension
did not improve and signs of
severe hypoxic brain damage
showed. Therapy was
minimized, pt was treated
symptomatically with
analgesic medication. She died
on 7/8/03.
Information regarding Prevnar
was received from a
healthcare professional via a
regulatory authority regarding
a 6 month old female pt who
experienced haemolytic
uraemic syndrome, renal
failure, haemolysis, anaemia,
LDH increased, platelet count
decreased, hypertension
arterial, serum creatinine
increased, hemoglobin
decreased, asthenia and pale.
The pt received a dose on
1/22/03. The pt experienced
asthenia and pale five days
after administration of
medication. anaemia was
diagnosed and she was
hospitalized. On 1/27/03
haemolytic uraemic syndrome
and renal failure were
diagnosed. The pt also
experienced haemolysis, LDH
increased, serum creatinine
increased and hemoglobin
decreased. After being
214912-1
611
months
HAEMOLYTIC
URAEMIC
Jan., 2003
SYNDROME
PNEUMO (PREVNAR)
(1001)
dismissed from hospital, she
again was hospitalized from
2/20/03 until 2/24/03 and from
2/27/03 until 5/14/03. She
experienced platelet count
decreased starting on 3/4/03.
Furthermore, hypertension
arterial was diagnosed. Due to
increasing serum creatinine,
peritoneal dialysis was started
on 3/2/03 after a Tenckhoff
catheter had been implanted.
Due to anaemia she was given
Erythropoietin. The
hypertension arterial was
difficult to treat. On 5/7/03 a
gastrostomy was performed
for alimentation. She was
dismissed from hospital on
5/14/03 and was treated on an
outpatient basis. On 5/31/03
pt experienced gasping for
breath and a emergency
physician performed
cardiopulmonary resuscitation.
She was admitted to an ICU.
Due to worsening of condition
and hypertension a bilateral
nephrectomy was performed
on 6/5/03. She was
transferred to a nephrological
unit on 6/23/03. Hypertension
did not improve and signs of
severe hypoxic brain damage
showed. Therapy was
minimized, pt was treated
symptomatically with
analgesic medication. She died
on 7/8/03.
Information regarding Prevnar
was received from a physician
regarding a 15 month old
female who received a dose of
Prevnar and subsequently
developed hemolyticuremic
syndrome and pneumococcal
pneumonia. The patient's
concurrent illness includes a
history of mild asthma.
Indication for Prevnar was
immunization. Product was
administered on an unspecified
date. Dose regimen was 1
dose (IM). Concomitant
medications were not
reported. Four days before
admission, a 15 month old
infant was seen by her
pediatrician for cough, fever,
and wheezing. She was
treated with inhaled steroids.
The following day, she was
again evaluated by the
pediatrician who ordered a
chest xray with the results of
right lung pneumonia. She was
then admitted. A chest
217931-1
HAEMOLYTIC
Unknown
12 years URAEMIC
Date
SYNDROME
PNEUMO (PREVNAR)
(1001)
computerized tomogram and
ultrasound were performed
and confirmed pneumonia. A
urine latex AG was performed
and the results indicated urine
pneumococcal antigen
positive. Day 2 of
hospitalization, videoassisted
thoracoscopic surgery was
performed; results were not
provided. The reporter
indicated that there were no
complications of surgery.
Treatment consisted of
cefuroxime, azithromycin,
methylprednisolone and
ampicillin sodium/sulbactam.
Day 3 of hospitalization
brought sudden death.
Laboratory tests performed at
resuscitation were:
hemoglobin, 0.6 g/dL; platelet
count, 20,000; white blood cell
count 18,000. The autopsy
reported cause of death was
hemolyticuremic syndrome.
The reporter indicated that this
was related to likely
pneumococcal infection. No
additional information was
available at the time of this
report.
It was reported in a published
article, title as stated above,
that an 8 month old white
male pt with complete factor H
deficiency and atypical
hemolytic uremic syndrome
(AHUS) who was vaccinated
with pneumococcal 23v
polysaccharide vaccine
(manufacturer unk) within a
few days experienced an aHUS
crisis. Molecular genetic
analysis found a point
mutation in position T12770A,
which introduces a premature
stop codon in CCP domain 15
At YE99Stop of factor H. Most
likely, this is the cause for a
defect in protein secretion. In
a time course investigation
after fresh frozen plasma
(FFP) infusion, a factor H half
life in plasma of about six
days was determined. Based
on this result, the pt was
treated successfully with
regular plasma infusions every
second week, and he
recovered completely. During
the course of a screening
program aimed at identifying
factor H dysfunction in aHUS,
a case of complete factor H
deficiency associated with
233756-1
237787-1
237787-1
611
months
< 6
months
< 6
months
HAEMOLYTIC
Unknown
URAEMIC
Date
SYNDROME
HAEMOLYTIC
URAEMIC
Mar., 2005
SYNDROME
HAEMOLYTIC
URAEMIC
Mar., 2005
SYNDROME
PNEUMO
(PNEUMOVAX) (30)
DTAP (INFANRIX)
(286)
HIB (PEDVAXHIB)
(129)
aHUS was found. The male pt,
first child of a healthy
consanguineous parents, was
born at 40 weeks of gestation
after an uneventful pregnancy.
Physical and mental
development were initially
normal. At eight months of
age, he presented with mild
periorbital edema after a short
episode of an unspecific
infectious disease with
diarrhea but without vomiting.
Laboratory investigations
found anemia (hemoglobin,
7.5 g/dl with elevated lactate
dehydrogenase(LDH;
maximum 689 U/L), mild
thrombocytopenia (platelets,
135 x 10^3/mcL (x 10^9/L)
and impairment of renal
function (serum creatinine, 1.1
mg/dL (97.2 mcmol/L); blood
urea nitrogen, 101 mg/dL
(36.1 mmol/L). Peripheral
blood smear was examined
but did not show
schistocytes/fragmentocytes.
For complement diagnosis,
serum and
ethylenediaminetraacetic acid
plasma were obtained at
different time points from the
pt and his parents and
immediately frozen and stored
at 80 degrees C until
analysis. Microbial
examinations of stool and
serum were negative for
enterohemorrhagic E coli
serotype 0157:H7.
Investigations for hep A, B and
C; cytomegalovirus; E
Vomiting, low grade fever,
anemia, electrolyte imbalance,
seizure like activity, and
cardiopulmonary arrest.
Received autopsy report which
revealed COD as hemolytic
uremic syndrome w/glomrulo
microthrombi & bilateral acute
interstitial nephritis. Other
findings at autopsy: cerebral
edema, hemolytic anemia,
acute renal failure, pulmonary
congestion c/w aspiration
pneumonia.
Vomiting, low grade fever,
anemia, electrolyte imbalance,
seizure like activity, and
cardiopulmonary arrest.
Received autopsy report which
revealed COD as hemolytic
uremic syndrome w/glomrulo
microthrombi & bilateral acute
interstitial nephritis. Other
findings at autopsy: cerebral
edema, hemolytic anemia,
acute renal failure, pulmonary
congestion c/w aspiration
pneumonia.
237787-1
237787-1
253465-1
< 6
months
< 6
months
HAEMOLYTIC
URAEMIC
Mar., 2005
SYNDROME
HAEMOLYTIC
URAEMIC
Mar., 2005
SYNDROME
HAEMOLYTIC
12 years URAEMIC
Jan., 2005
SYNDROME
PNEUMO (PREVNAR)
(1001)
Vomiting, low grade fever,
anemia, electrolyte imbalance,
seizure like activity, and
cardiopulmonary arrest.
Received autopsy report which
revealed COD as hemolytic
uremic syndrome w/glomrulo
microthrombi & bilateral acute
interstitial nephritis. Other
findings at autopsy: cerebral
edema, hemolytic anemia,
acute renal failure, pulmonary
congestion c/w aspiration
pneumonia.
POLIO VIRUS, INACT.
(IPOL) (1030)
Vomiting, low grade fever,
anemia, electrolyte imbalance,
seizure like activity, and
cardiopulmonary arrest.
Received autopsy report which
revealed COD as hemolytic
uremic syndrome w/glomrulo
microthrombi & bilateral acute
interstitial nephritis. Other
findings at autopsy: cerebral
edema, hemolytic anemia,
acute renal failure, pulmonary
congestion c/w aspiration
pneumonia.
MEASLES + MUMPS
+ RUBELLA (MMR II)
(26)
Information has been received
from a health authority
(reference #ADROIT S003403)
concerning a 12 month old
female with a history of
recurrent chest infection who
on 10Jan2005 was
vaccinated intramuscularly
with a 0.5 mL dose of measles
virus vaccine live (+) mumps
virus vaccine live (+) rubella
virus vaccine live. The patient
received oral amoxicillin for
pyrexia and a cough from 16
Jan2005 to 18Jan2005,
intravenous amoxicillin (+)
clavulanate potassium (900
mg) for an unknown indication
from 18Jan2005 to 21Jan
2005, and intravenous
furosemide from 18Jan2005
to 21Jan2005. On 21Jan
2005, 11 days after
vaccination, the patient
developed atypical hemolytic
uremic syndrome. The patient
received treatment with
hemofiltration and
plasmapheresis. The patient
recovered on 25Feb2005.
The reporter and the health
authority considered this to be
a serious reaction as it
involved or prolonged
hospitalization and was
considered to be life
threatening. No further
information is available and
the case is closed. Other
business partner number
include E200601398.
273021-1
275514-1
HAEMOLYTIC
Unknown
12 years URAEMIC
Date
SYNDROME
HAEMOLYTIC
12 years URAEMIC
Oct., 2006
SYNDROME
PNEUMO (PREVNAR)
(1001)
PNEUMO (PREVNAR)
(1001)
Information regarding Prevnar
was received from a
healthcare professional
regarding a 30monthold
female patient who
experienced meningitis, facial
palsy and uremic hemolytic
syndrome. The patient
received the fourth dose on an
unspecified date. On 31Jan
2007 the patient was
hospitalized with meningitis
(drug ineffective). On 02Feb
2007 she developed facial
palsy and uremic hemolytic
syndrome. The patient was
treated with unspecified
antibiotics for the meningitis,
no specific therapy was given
for the other events. She
recovered from the meningitis
and uremic syndrome.
Recovery from the facial palsy
is unknown. Culture (results
unspecified cultures
negative) and blood culture
(results: negative) were done
on 31Jan2007. Urine analysis
(results; positive for
pneumococcal antigen) was
done on 02Feb2007. No
additional information was
available at the time of this
report.
Information regarding Prevnar
was received from a
healthcare professional
regarding a 21monthold
female patient who
experienced pneumococcal
pneumonia complicated with
hemolytic uremic syndrome.
The patient received the fourth
dose on 10Oct2006. The
patient experienced
pneumococcal pneumonia
complicated with hemolytic
uremic syndrome on 03Feb
2007. The patient was
hospitalised. Pneumococcal
serotype 19 A was identified.
The events were considered to
be lifethreatening. At the time
of reporting. the patient was
still hospitalized, however the
patient's health status had
improved, dialysis had been
stopped and she was
Download VAERS Results Form shu
VAERS Results Form shu.pdf (PDF, 546.08 KB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000353890.