direct download complete solution of h c verma (PDF)
File information
This PDF 1.6 document has been generated by Nitro PDF 5.1 / BCL easyPDF 5.10 (0420), and has been sent on pdf-archive.com on 09/05/2016 at 16:04, from IP address 117.247.x.x.
The current document download page has been viewed 631 times.
File size: 1.96 MB (232 pages).
Privacy: public file



File preview
CHAPTER 24
KINETIC THEORY OF GASES
1.
Volume of 1 mole of gas
RT
0.082 273
–3
–2
3
PV = nRT V =
=
= 22.38 ≈ 22.4 L = 22.4 × 10 = 2.24 × 10 m
P
1
2.
n=
3.
click
here
1 1 10 3
10 3
PV
1
=
=
=
RT
0.082 273
22.4
22400
1
23
19
No of molecules = 6.023 × 10 ×
= 2.688 × 10
22400
3
–5
V = 1 cm ,
T = 0°C,
P = 10 mm of Hg
1.36 980 10 6 1
PV
ƒgh V
–13
=
=
= 5.874 × 10
RT
RT
8.31 273
23
–13
11
No. of moluclues = No × n = 6.023 × 10 × 5.874 × 10 = 3.538 × 10
n=
4.
n=
1 1 10 3
10 3
PV
=
=
RT
0.082 273
22.4
5.
10
3
32
–3
g = 1.428 × 10 g = 1.428 mg
22.4
Since mass is same
n1 = n2 = n
nR 300
nR 600
P1 =
,
P2 =
V0
2V0
mass =
2V0
2V0
P1
nR 300
1
=
= =1:1
P2
V0
nR 600 1
6.
600 K
–3
V = 250 cc = 250 × 10
–3
–3
–3
–6
–3
P = 10 mm = 10 × 10 m = 10 × 13600 × 10 pascal = 136 × 10 pascal
T = 27°C = 300 K
136 10 3 250
PV
136 250
=
10 3 =
10 6
8.3 300
RT
8.3 300
136 250
No. of molecules =
10 6 6 10 23 = 81 × 1017 ≈ 0.8 × 1015
8.3 300
5
6
P1 = 8.0 × 10 Pa,
P2 = 1 × 10 Pa,
T1 = 300 K,
Since, V1 = V2 = V
n=
7.
8.
9.
T2 = ?
P1V1
PV
8 10 5 V
1 10 6 300
1 10 6 V
= 2 2
=
T2 =
= 375° K
T1
T2
300
T2
8 10 5
3
6
3
T = 300 K,
P=?
m = 2 g, V = 0.02 m = 0.02 × 10 cc = 0.02 × 10 L,
M = 2 g,
m
2
PV = nRT PV =
RT P × 20 = 0.082 300
M
2
0.082 300
5
5
P=
= 1.23 atm = 1.23 × 10 pa ≈ 1.23 × 10 pa
20
nRT
m RT
ƒRT
P=
=
=
V
M V
M
–3
3
ƒ 1.25 × 10 g/cm
7
R 8.31 × 10 ert/deg/mole
T 273 K
M=
ƒRT
1.25 10 3 8.31 10 7 273
4
=
= 0.002796 × 10 ≈ 28 g/mol
P
13.6 980 76
24.1
V0
300 K
Kinetic Theory of Gases
10. T at Simla = 15°C = 15 + 273 = 288 K
–2
P at Simla = 72 cm = 72 × 10 × 13600 × 9.8
T at Kalka = 35°C = 35 + 273 = 308 K
–2
P at Kalka = 76 cm = 76 × 10 × 13600 × 9.8
PV = nRT
PM
m
m
RT PM =
RT ƒ =
PV =
RT
M
V
PSimla M RTKalka
ƒSimla
=
ƒKalka
RTSimla
PKalka M
=
72 10 2 13600 9.8 308
2
288 76 10 13600 9.8
1
ƒKalka
=
= 0.987
ƒSimla
1.013
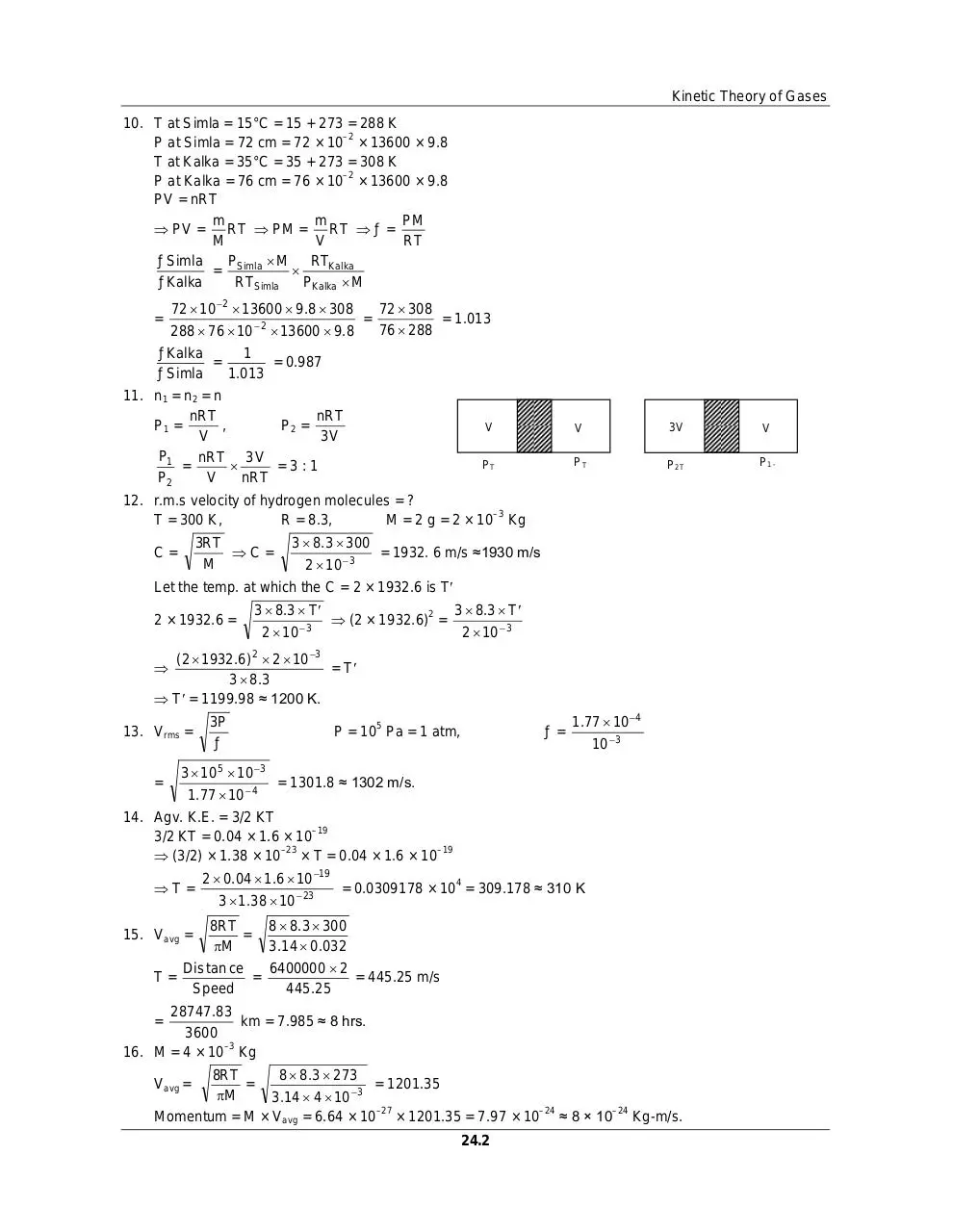
11. n1 = n2 = n
nRT
nRT
,
P2 =
P1 =
V
3V
P1
nRT 3 V
=
=3:1
P2
V
nRT
=
72 308
= 1.013
76 288
V
V
3V
V
PT
PT
P2T
P1 -
12. r.m.s velocity of hydrogen molecules = ?
–3
T = 300 K,
R = 8.3,
M = 2 g = 2 × 10 Kg
3RT
C=
M
C=
3 8.3 300
2 10 3
= 1932. 6 m/s ≈1930 m/s
Let the temp. at which the C = 2 × 1932.6 is T
2 × 1932.6 =
3 8 .3 T
2 10
3
(2 × 1932.6) =
2
3 8 .3 T
2 10 3
(2 1932.6)2 2 10 3
= T
3 8 .3
T = 1199.98 ≈ 1200 K.
13. Vrms =
3P
ƒ
5
P = 10 Pa = 1 atm,
ƒ=
1.77 10 4
10 3
3 10 5 10 3
= 1301.8 ≈ 1302 m/s.
1.77 10 4
14. Agv. K.E. = 3/2 KT
–19
3/2 KT = 0.04 × 1.6 × 10
–23
–19
(3/2) × 1.38 × 10 × T = 0.04 × 1.6 × 10
=
T=
2 0.04 1.6 10 19
3 1.38 10 23
4
= 0.0309178 × 10 = 309.178 ≈ 310 K
8RT
8 8.3 300
=
M
3.14 0.032
Dis tan ce
6400000 2
T=
=
= 445.25 m/s
Speed
445 .25
15. Vavg =
28747 .83
km = 7.985 ≈ 8 hrs.
3600
–3
16. M = 4 × 10 Kg
=
8 8.3 273
8RT
=
= 1201.35
M
3.14 4 10 3
–27
–24
–24
Momentum = M × Vavg = 6.64 × 10 × 1201.35 = 7.97 × 10 ≈ 8 × 10 Kg-m/s.
Vavg =
24.2
Kinetic Theory of Gases
8RT
8 8.3 300
=
M
3.14 0.032
8RT1
8RT2
Now,
=
2
4
17. Vavg =
T1
1
=
T2
2
8RT
M
18. Mean speed of the molecule =
Escape velocity =
8RT
=
M
T=
VavgN2
2gr
8RT
= 2gr
M
2grM
2 9.8 6400000 3.14 2 10 3
=
= 11863.9 ≈ 11800 m/s.
8R
8 8 .3
8RT
M
19. Vavg =
VavgH2
2gr
=
8RT
28
=
2
8RT
28
=
2
14 = 3.74
20. The left side of the container has a gas, let having molecular wt. M1
Right part has Mol. wt = M2
Temperature of both left and right chambers are equal as the separating wall is diathermic
3RT
=
M1
21. Vmean =
3RT
8RT
8RT
=
M1
M2
M2
8RT
=
M
M1
M
3
3
=
1 =
= 1.1775 ≈ 1.18
M2
M2
8
8
8 8.3 273
= 1698.96
3.14 2 10 3
Total Dist = 1698.96 m
1698.96
10
= 1.23 × 10
No. of Collisions =
1.38 10 7
5
22. P = 1 atm = 10 Pascal
–3
T = 300 K,
M = 2 g = 2 × 10 Kg
8RT
8 8.3 300
=
= 1781.004 ≈ 1780 m/s
M
3.14 2 10 3
(b) When the molecules strike at an angle 45°,
(a) Vavg =
Force exerted = mV Cos 45° – (–mV Cos 45°) = 2 mV Cos 45° = 2 m V
No. of molecules striking per unit area =
=
10
5
2 2 10
3
1780
=
3
2 1780
Force
2mv Area
=
1
2
=
2 mV
Pr essure
2mV
10 31 = 1.19 × 10–3 × 1031 = 1.19 × 1028 ≈ 1.2 × 1028
6 10 23
PV
PV
23. 1 1 = 2 2
T1
T2
P1 200 KPa = 2 × 10 pa
T1 = 20°C = 293 K
102 V1
V2 = V1 + 2% V1 =
100
5
P2 = ?
T2 = 40°C = 313 K
P 102 V1
2 10 5 V1
2 10 7 313
= 2
P2 =
= 209462 Pa = 209.462 KPa
293
100 313
102 293
24.3
Kinetic Theory of Gases
–3
3
5
24. V1 = 1 × 10 m ,
P1V1 = n1R1T1
n=
P1 = 1.5 × 10 Pa,
P1V1
1.5 10 5 1 10 3
=
R1T1
8.3 400
T1 = 400 K
n=
1 .5
8 .3 4
1 .5
1 .5
M =
32 = 1.4457 ≈ 1.446
8 .3 4
8 .3 4
5
–3
3
V2 = 1 × 10 m ,
P2 = 1 × 10 Pa,
P2V2 = n2R2T2
m1 =
n2 =
T2 = 300 K
P2 V2
10 5 10 3
1
=
=
= 0.040
R 2 T2
8.3 300
3 8 .3
m2 = 0.04 × 32 = 1.285
m = m1 – m2 =1.446 – 1.285 = 0.1608 g ≈ 0.16 g
5
5
5
25. P1 = 10 + ƒgh = 10 + 1000 × 10 × 3.3 = 1.33 × 10 pa
4
5
–3 3
T1 = T2 = T,
V1 = (2 × 10 )
P2 = 10 ,
3
4 3
V2 = r ,
r=?
3
P1V1
PV
= 2 2
T1
T2
1.33 10 5
4
4
(2 10 3 )3
10 5 r 2
3
3
=
T1
T2
1.33 × 8 × 10 × 10 = 10 × r
5
26. P1 = 2 atm = 2 × 10 pa
3
T1 = 300 K
V1 = 0.002 m ,
P1V1 = n1RT1
5
n=
–9
5
r=
3
3
–3
10.64 10 3 = 2.19 × 10 ≈ 2.2 mm
P1V1 2 10 5 0.002
4
=
= 0.1606
=
RT1
8.3 300
8 .3 3
5
P2 = 1 atm = 10 pa
3
V2 = 0.0005 m ,
P2V2 = n2RT2
n2 =
T2 = 300 K
P2 V2
5
1
10 5 0.0005
=
=
= 0.02
RT2
8.3 300
3 8.3 10
n = moles leaked out = 0.16 – 0.02 = 0.14
27. m = 0.040 g,
T = 100°C,
MHe = 4 g
3
3 m
T = ?
U = nRt = RT
2
2 M
3 m
3 m
Given RT 12 = RT
2 M
2 M
1.5 × 0.01 × 8.3 × 373 + 12 = 1.5 × 0.01 × 8.3 × T
58.4385
= 469.3855 K = 196.3°C ≈ 196°C
T =
0.1245
2
28. PV = constant
2
2
P1V1 = P2V2
nRT1
nRT2
V12 =
V2 2
V1
V2
T1 V1 = T2 V2 = TV = T1 × 2V T2 =
T
2
24.4
Kinetic Theory of Gases
29. PO2 =
nO2
no2 RT
,
PH2 =
nH2 RT
V
V
1.60
m
=
=
= 0.05
32
MO2
nO nH2
RT
Now, Pmix = 2
V
2.80
m
nH2 =
=
= 0.1
MH2
28
(0.05 0.1) 8.3 300
2
= 2250 N/m
0.166
30. P1 = Atmospheric pressure = 75 × ƒg
V1 = 100 × A
P2 = Atmospheric pressure + Mercury pessue = 75ƒg + hgƒg (if h = height of mercury)
V2 = (100 – h) A
P1V1 = P2V2
75ƒg(100A) = (75 + h)ƒg(100 – h)A
2
75 × 100 = (74 + h) (100 – h) 7500 = 7500 – 75 h + 100 h – h
2
2
h – 25 h = 0 h = 25 h h = 25 cm
Height of mercury that can be poured = 25 cm
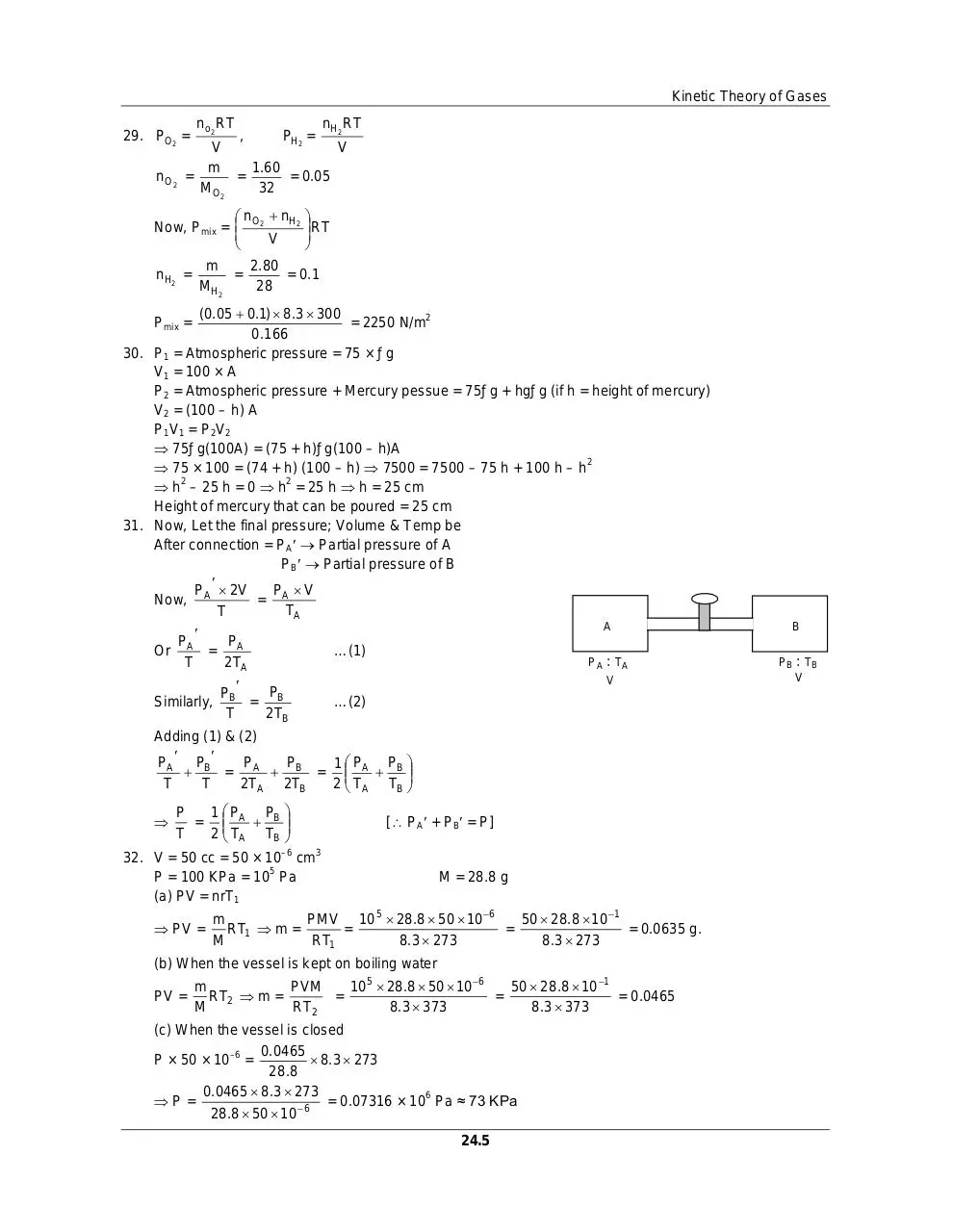
31. Now, Let the final pressure; Volume & Temp be
After connection = PA Partial pressure of A
PB Partial pressure of B
P 2V
P V
Now, A
= A
TA
T
Pmix =
P
P
Or A = A
T
2TA
A
…(1)
PA
Similarly,
P
PB
= B
2TB
T
: TA
V
…(2)
Adding (1) & (2)
PA PB
P
P
P
1P
= A B = A B
2TA 2TB
T
T
2 TA TB
P
P
1P
= A B
T
2 TA TB
32. V = 50 cc = 50 × 10–6 cm3
5
P = 100 KPa = 10 Pa
(a) PV = nrT1
PV =
[ PA + PB = P]
M = 28.8 g
m
PMV 10 5 28.8 50 10 6
50 28.8 10 1
RT1 m =
=
=
= 0.0635 g.
RT1
8.3 273
8.3 273
M
(b) When the vessel is kept on boiling water
PV =
PVM
10 5 28.8 50 10 6
50 28.8 10 1
m
RT2 m =
=
=
= 0.0465
RT2
8.3 373
8.3 373
M
(c) When the vessel is closed
0.0465
–6
P × 50 × 10 =
8.3 273
28.8
0.0465 8.3 273
6
P=
= 0.07316 × 10 Pa ≈ 73 KPa
28.8 50 10 6
24.5
B
PB
: TB
V
Kinetic Theory of Gases
33. Case I Net pressure on air in volume V
= Patm – hƒg = 75 × ƒHg – 10 ƒHg = 65 × ƒHg × g
Case II Net pressure on air in volume ‘V’ = Patm + ƒHg × g × h
P1V1 = P2V2
ƒHg × g × 65 × A × 20 = ƒHg × g × 75 + ƒHg × g × 10 × A × h
65 20
= 15.2 cm ≈ 15 cm
62 × 20 = 85 h h =
85
34. 2L + 10 = 100 2L = 90 L = 45 cm
Applying combined gas eqn to part 1 of the tube
( 45 A )P0
( 45 x )P1
=
300
273
273 45 P0
P1 =
300( 45 x )
Applying combined gas eqn to part 2 of the tube
45 AP0
( 45 x )AP2
=
300
400
400 45 P0
P2 =
300( 45 x )
P1 = P2
273 45 P0
400 45 P0
=
300( 45 x )
300( 45 x )
V
20 cm
P 20 A
P A
=
…(1)
400
T
P 10 A
P(30 x )
=
…(2)
100
T
Equating (1) and (2)
1
x
=
30 – x = 2x 3x = 30 x = 10 cm
2
30 x
The separator will be at a distance 10 cm from left end.
24.6
h
V
27°C
L
l
1
2
P0
10
P0
L-x
L+x
P1
P2
0°C
(45 – x) 400 = (45 + x) 273
18000 – 400 x = 12285 + 273 x
(400 + 273)x = 18000 – 12285 x = 8.49
273 46 76
P1 =
= 85 % 25 cm of Hg
300 36.51
Length of air column on the cooler side = L – x = 45 – 8.49 = 36.51
35. Case I Atmospheric pressure + pressure due to mercury column
Case II Atmospheric pressure + Component of the pressure due
to mercury column
20cm
P1V1 = P2V2
43cm
(76 × ƒHg × g + ƒHg × g × 20) × A × 43
= (76 × ƒHg × g + ƒHg × g × 20 × Cos 60°) A × ℓ
96 × 43 = 86 × ℓ
96 43
= 48 cm
ℓ=
86
36. The middle wall is weakly conducting. Thus after a long
10 cm
20 cm
time the temperature of both the parts will equalise.
The final position of the separating wall be at distance x
400 K
100 K
P
from the left end. So it is at a distance 30 – x from the right
P
end
Putting combined gas equation of one side of the separating wall,
P1 V1
P V2
= 2
T1
T2
10 cm
10 cm
27°C
10
0°C
60°
ℓ
x
T P
30 – x
T P
Kinetic Theory of Gases
37.
dV
= r dV = r dt
dt
Let the pumped out gas pressure dp
Volume of container = V0 At a pump dv amount of gas has been pumped out.
Pdv = –V0df PV df = –V0 dp
P
P
dp
=
p
t
dtr
V
0
P = P e rt / V0
0
Half of the gas has been pump out, Pressure will be half =
ln 2 =
38. P =
rt
V0
t = ln2
1 vt / V0
e
2
0
r
P0
V
1
V0
nRT
=
V
RT
=
V
RT
=
V0
2
P0
V
1
V0
P0
V
1
V0
P0
2
V
1
V0
2
2
[PV = nRT according to ideal gas equation]
[Since n = 1 mole]
[At V = V0]
P0V0 = RT(1 +1) P0V0 = 2 RT T =
P0 V0
2R
39. Internal energy = nRT
Now, PV = nRT
PV
Here P & V constant
nT =
R
nT is constant
Internal energy = R × Constant = Constant
40. Frictional force = N
Let the cork moves to a distance = dl
Work done by frictional force = Nde
Before that the work will not start that means volume remains constant
P
P
P
1
= 2 P2 = 2 atm
1 = 2
T1
T2
300
600
Extra Pressure = 2 atm – 1 atm = 1 atm
Work done by cork = 1 atm (Adl)
Ndl = [1atm][Adl]
1 10 5 (5 10 2 )2
1 10 5 25 10 5
=
2
2
dN
N
Total circumference of work = 2r
=
dl
2r
N=
=
1 10 5 25 10 5
1 10 5 25 10 5
4
=
= 1.25 × 10 N/M
0.2 2r
0.2 2 5 10 5
24.7
Kinetic Theory of Gases
41.
P1V1
PV
= 2 2
T1
T2
2P0
P0
P0 V
PV
=
P = 2 P0
T0
2T0
Net pressure = P0 outwards
Tension in wire = P0 A
Where A is area of tube.
[ Since liquid at the same level have same pressure]
42. (a) 2P0x = (h2 + h0)ƒg
2P0 = h2 ƒg + h0 ƒg
h2
h2 ƒg = 2P0 – h0 ƒg
2P0
2P0
2P0 h 0 ƒg
=
h0
h2 =
ƒg
ƒg
ƒg
(b) K.E. of the water = Pressure energy of the water at that layer
P
1
2
mV = m
2
ƒ
V =
2
2P
2
=
ƒ
ƒP0 ƒg(h1 h 0
1/ 2
2
V=
ƒ
P
ƒ
g
(
h
h
0
1
0
(c) (x + P0)ƒh = 2P0
2P0 + ƒg (h –h0)= P0 + ƒgx
P0
= h2 + h1
X=
ƒg h1 h0
i.e. x is h1 meter below the top x is –h1 above the top
43. A = 100 cm2 = 10–3 m
m = 1 kg,
P = 100 K Pa = 105 Pa
ℓ = 20 cm
Case I = External pressure exists
Case II = Internal Pressure does not exist
P1V1 = P2V2
1 9 .8
1 9 .8
10 5
× V
V =
3
10 3
10
(10 + 9.8 × 10 )A × ℓ = 9.8 × 10 × A × ℓ
5
–1
2
3
10 × 2 × 10 + 2 × 9.8 × 10 = 9.8 × 10 × ℓ
5
ℓ =
3
2 10 4 19.6 10 2
9.8 10 3
3
= 2.24081 m
44. P1V1 = P2V2
mg
P0 A P0 Aℓ
A
1 9. 8
10 5 0.2 = 105 ℓ
10 10 4
(9.8 × 10 + 10 )× 0.2 = 10 ℓ
3
5
109.8 × 10 × 0.2 = 10 ℓ
109.8 0.2
= 0.2196 ≈ 0.22 m ≈ 22 cm
ℓ =
10 2
3
5
5
24.8
h0
h1
Kinetic Theory of Gases
45. When the bulbs are maintained at two different temperatures.
The total heat gained by ‘B’ is the heat lost by ‘A’
Let the final temp be x
So, m1 St = m2 St
n1 x = 62n2 – n2 x
n1 M × s(x – 0) = n2 M × S × (62 – x)
x=
V
V
A
B
62n 2
62n 2
=
= 31°C = 304 K
n1 n 2
2n 2
For a single ball
P1V1
PV
= 2 2
T1
T2
Initial Temp = 0°C
P = 76 cm of Hg
V1 = V2
Hence n1 = n2
P V
76 V
403 76
= 2
P2 =
= 84.630 ≈ 84°C
273
304
273
46. Temp is 20°
Relative humidity = 100%
So the air is saturated at 20°C
Dew point is the temperature at which SVP is equal to present vapour pressure
So 20°C is the dew point.
47. T = 25°C
P = 104 KPa
VP
[SVP = 3.2 KPa,
RH = 0.6]
SVP
3
3
3
VP = 0.6 × 3.2 × 10 = 1.92 × 10 ≈ 2 × 10
When vapours are removed VP reduces to zero
Net pressure inside the room now = 104 × 103 – 2 × 103 = 102 × 103 = 102 KPa
48. Temp = 20°C
Dew point = 10°C
The place is saturated at 10°C
Even if the temp drop dew point remains unaffected.
The air has V.P. which is the saturation VP at 10°C. It (SVP) does not change on temp.
RH =
VP
SVP
The point where the vapour starts condensing, VP = SVP
We know P1V1 = P2V2
3
RH SVP × 10 = SVP × V2
V2 = 10RH 10 × 0.4 = 4 cm
50. Atm–Pressure = 76 cm of Hg
When water is introduced the water vapour exerts some pressure which counter acts the atm pressure.
The pressure drops to 75.4 cm
Pressure of Vapour = (76 – 75.4) cm = 0.6 cm
49. RH =
VP
0 .6
=
= 0.6 = 60%
SVP
1
51. From fig. 24.6, we draw r, from Y axis to meet the graphs.
Hence we find the temp. to be approximately 65°C & 45°C
52. The temp. of body is 98°F = 37°C
At 37°C from the graph SVP = Just less than 50 mm
B.P. is the temp. when atmospheric pressure equals the atmospheric pressure.
Thus min. pressure to prevent boiling is 50 mm of Hg.
53. Given
SVP at the dew point = 8.9 mm
SVP at room temp = 17.5 mm
Dew point = 10°C as at this temp. the condensation starts
Room temp = 20°C
R. Humidity =
RH =
8 .9
SVP at dew po int
=
= 0.508 ≈ 51%
SVP at room temp
17.5
24.9
Download direct download complete solution of h c verma
direct download complete solution of h c verma.pdf (PDF, 1.96 MB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000370177.