B742 02 QP Jun15 (PDF)
File information
Title: B742-02Jun15_88242.indd
Author: lkeith
This PDF 1.6 document has been generated by Adobe InDesign CS4 (6.0.6) / Acrobat Distiller 9.0.0 (Macintosh), and has been sent on pdf-archive.com on 19/06/2016 at 14:54, from IP address 90.199.x.x.
The current document download page has been viewed 744 times.
File size: 754.63 KB (32 pages).
Privacy: public file




File preview
H
Oxford Cambridge and RSA
Monday 15 June 2015 – Morning
GCSE GATEWAY SCIENCE
CHEMISTRY B
B742/02 Chemistry modules C4, C5, C6 (Higher Tier)
* 3 7 7 5 4 0 4 3 6 2 *
Candidates answer on the Question Paper.
A calculator may be used for this paper.
Duration: 1 hour 30 minutes
OCR supplied materials:
None
Other materials required:
•
Pencil
•
Ruler (cm/mm)
*
B
7
4
2
0
2
*
INSTRUCTIONS TO CANDIDATES
•
•
•
•
•
•
Write your name, centre number and candidate number in the boxes above. Please write
clearly and in capital letters.
Use black ink. HB pencil may be used for graphs and diagrams only.
Answer all the questions.
Read each question carefully. Make sure you know what you have to do before starting
your answer.
Write your answer to each question in the space provided. Additional paper may be
used if necessary but you must clearly show your candidate number, centre number and
question number(s).
Do not write in the bar codes.
INFORMATION FOR CANDIDATES
•
•
•
•
•
Your quality of written communication is assessed in questions marked with a pencil (
The Periodic Table can be found on the back page.
The number of marks is given in brackets [ ] at the end of each question or part
question.
The total number of marks for this paper is 85.
This document consists of 32 pages. Any blank pages are indicated.
© OCR 2015 [D/601/6476]
DC (LK/SW) 88242/4
).
OCR is an exempt Charity
Turn over
2
Answer all the questions.
SECTION A – Module C4
1
Look at the electronic structures of some atoms.
(a) (i)
Atom
Electronic structure
W
2.8.1
X
2.8.4
Y
2.8.7
Z
2.8.8
One of the atoms is a metal which makes a positive ion.
Which one? Choose from the table.
answer ........................................
(ii)
[1]
One of the atoms has a stable electronic structure and is unreactive.
Which one? Choose from the table.
answer ........................................
(iii)
[1]
Two of the atoms can combine together by transferring electrons to form an
ionic bond.
Which two? Choose from the table.
.......................................... and ..........................................
© OCR 2015
[1]
3
(b) Ammonia has the formula, NH3.
The electronic structure of nitrogen is 2.5.
The electronic structure of hydrogen is 1.
Draw a ‘dot and cross’ diagram to show the covalent bonding in ammonia.
Show all the electrons.
[2]
(c) Sodium chloride is an ionic compound.
Sodium chloride
•
will not conduct electricity when it is a solid
•
will conduct electricity when it is dissolved in water.
Explain these two observations in terms of structure and bonding.
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
.............................................................................................................................................. [2]
© OCR 2015
Turn over
4
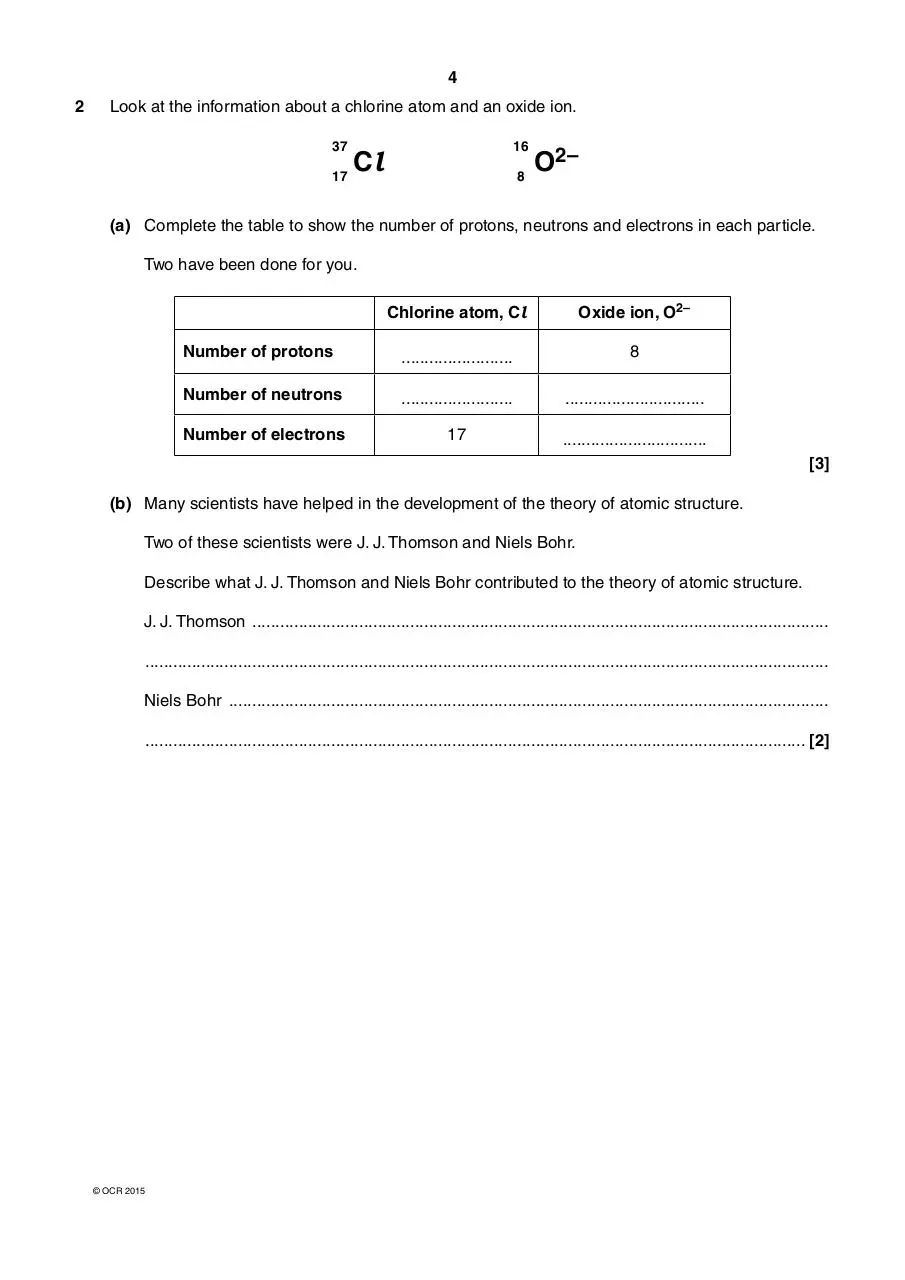
2
Look at the information about a chlorine atom and an oxide ion.
37
17
16
Cl
8
O2–
(a) Complete the table to show the number of protons, neutrons and electrons in each particle.
Two have been done for you.
Chlorine atom, Cl
Oxide ion, O2–
Number of protons
........................
8
Number of neutrons
........................
..............................
Number of electrons
17
...............................
[3]
(b) Many scientists have helped in the development of the theory of atomic structure.
Two of these scientists were J. J. Thomson and Niels Bohr.
Describe what J. J. Thomson and Niels Bohr contributed to the theory of atomic structure.
J. J. Thomson ............................................................................................................................
...................................................................................................................................................
Niels Bohr .................................................................................................................................
.............................................................................................................................................. [2]
© OCR 2015
5
BLANK PAGE
PLEASE DO NOT WRITE ON THIS PAGE
© OCR 2015
Turn over
6
3
This question is about the reaction of Group 1 elements with water.
Lithium, sodium and potassium are Group 1 elements.
They all react with water.
Group 1 elements
water
Look at the table.
Group 1 element
Observations
15
melts
moves across surface of water
makes a gas which burns with a ‘pop’
makes an alkaline solution
potassium
7
melts and catches fire
moves quickly across surface of water
makes a gas which burns with a ‘pop’
makes an alkaline solution
lithium
25
moves slowly across surface of water
makes a gas which burns with a ‘pop’
makes an alkaline solution
sodium
© OCR 2015
Time for 0.5 g of
metal to react
in seconds
7
Rubidium is another Group 1 element.
It is below potassium in Group 1 of the periodic table.
Predict the reaction time, and name the products, of the reaction between rubidium and water.
Include a balanced symbol equation for the reaction.
The quality of written communication will be assessed in your answer to this question.
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..........................................................................................................................................................
..................................................................................................................................................... [6]
© OCR 2015
Turn over
8
4
This question is about substances that are found in different types of water.
(a) River water contains dissolved substances.
River water has to be purified before it can be drunk.
The water purification process has three stages.
These are
•
filtration
•
sedimentation
•
chlorination.
Pollutants such as fertilisers are still in the water after this purification.
Explain why.
...................................................................................................................................................
.............................................................................................................................................. [1]
(b) Sea water can be made into drinking water.
One way this can be done is by distillation.
Look at the diagram. It shows the apparatus used to distil water in the laboratory.
water out
condenser
sea water
water in
heat
fresh water
Explain the disadvantages of using distillation to make large amounts of drinking water.
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
.............................................................................................................................................. [2]
© OCR 2015
9
(c) Pete analyses two samples.
Look at Pete’s results.
Sample
Addition of
sodium hydroxide solution
Addition of
barium chloride solution
A
blue solid made
white solid made
B
brown solid made
no reaction
Pete thinks that sample A is copper sulfate.
He thinks that sample B is iron(III) sulfate.
Is Pete right about each sample?
Explain your answer.
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
...................................................................................................................................................
.............................................................................................................................................. [4]
© OCR 2015
Turn over
Download B742-02 QP Jun15
B742-02 QP Jun15.pdf (PDF, 754.63 KB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000390283.