Atomic Structure and Interatomic Bonding(2) (PDF)
File information
Title: PowerPoint Presentation
Author: Campbell
This PDF 1.5 document has been generated by Microsoft® PowerPoint® 2013, and has been sent on pdf-archive.com on 19/07/2016 at 23:08, from IP address 73.42.x.x.
The current document download page has been viewed 715 times.
File size: 1.68 MB (37 pages).
Privacy: public file



File preview
Materials Science and
Engineering
Atomic Structure and Interatomic
Bonding
1
Atomic Structure
• Nucleus composed of proton and neutrons,
surrounded by moving electrons.
• Proton has positive charge (+1.602 x 10-19 C)
• Neutron has no charge
• Electron has negative charge (-1.602 x 10-19 C)
• For a neutral atom the number of protons is equal
to the number of electrons.
• Each chemical element is characterized by the
number of protons in the nucleus or the atomic
number (Z).
• Atomic mass (A) is the sum of the masses of
protons and neutrons in the nucleus.
Fig. 2.1 Schematic representation
of the Bohr atom (from Textbook)
2
Atomic Structure
• The number of protons is the same for all atoms of a given element.
• The number of neutrons in the atom a given element may be variable,
resulting in some elements having two or more atomic masses, called
isotopes.
• The atomic weight of an element is the weighted average of the
atomic masses of the element’s naturally occurring isotopes.
• One atomic mass unit (1 amu) = 1/12 of the atomic mass of the
carbon 12 isotope which has an atomic mass (A) of 12.00000 amu.
3
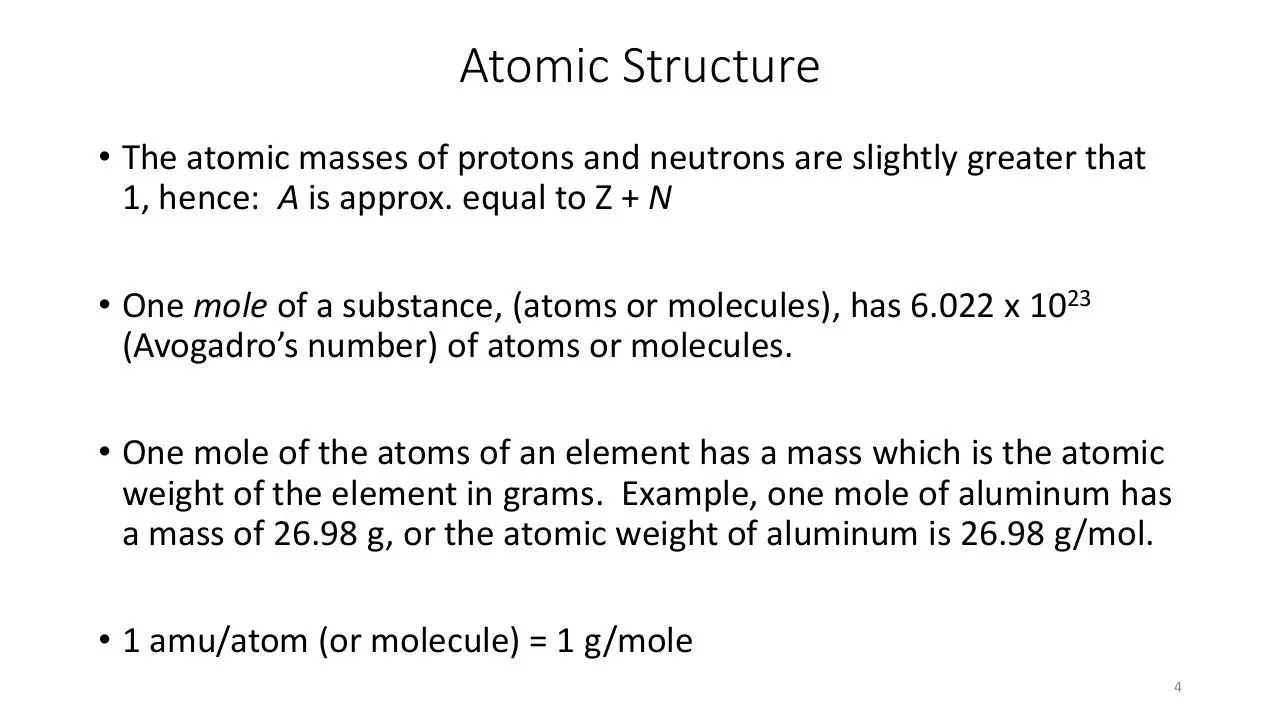
Atomic Structure
• The atomic masses of protons and neutrons are slightly greater that
1, hence: A is approx. equal to Z + N
• One mole of a substance, (atoms or molecules), has 6.022 x 1023
(Avogadro’s number) of atoms or molecules.
• One mole of the atoms of an element has a mass which is the atomic
weight of the element in grams. Example, one mole of aluminum has
a mass of 26.98 g, or the atomic weight of aluminum is 26.98 g/mol.
• 1 amu/atom (or molecule) = 1 g/mole
4
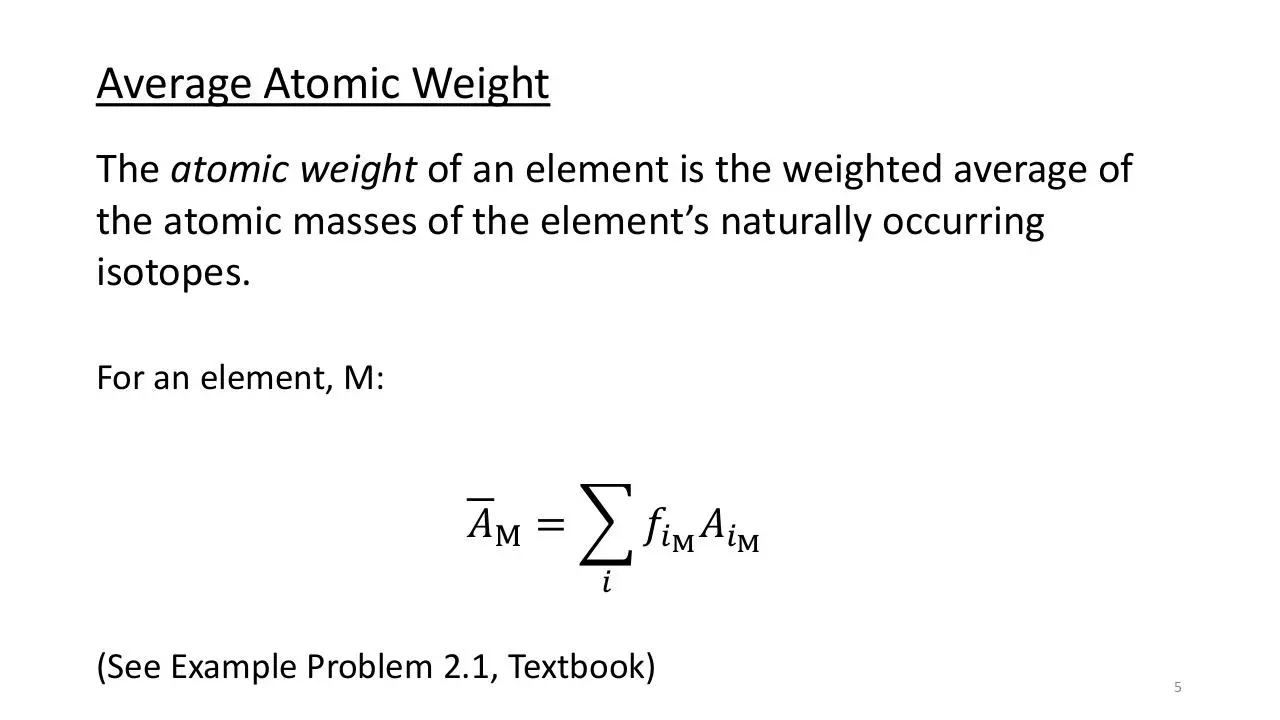
Average Atomic Weight
The atomic weight of an element is the weighted average of
the atomic masses of the element’s naturally occurring
isotopes.
For an element, M:
𝐴M =
𝑓𝑖M 𝐴𝑖M
𝑖
(See Example Problem 2.1, Textbook)
5
Atomic Structure
• Base on quantum mechanics, (1)
electrons can only occupy discrete
orbitals of specified energy levels
around an atom, (2) the energies of
electrons are quantized. That is,
electrons can only have specific
values of energy.
• An electron may change it energy,
but to do so, it must make a
quantum jump to an allowed
higher energy level or to an
allowed lower energy level.
6
Electron Quantum Numbers
• Every electron is characterized by four quantum numbers (n, l, ml and
ms).
• Principal quantum number, n. n = 1, 2, 3, 4, 5, … or designated by
letters K, L, M, N, O, ….
• Second (azimuthal) quantum number, l, designates the subshells. Can
only have values of l = 0 to l = (n-1). Also designated by letters, as
follows:
7
Electron Quantum Numbers
• Magnetic quantum number, ml. ml can have values between –l and +l,
including 0.
• Spin moment quantum number, ms. Two possible values: +1/2 (spin
up) and -1/2 (spin down).
(See table on next slide)
8
Electron Quantum Numbers
9
Download Atomic Structure and Interatomic Bonding(2)
Atomic Structure and Interatomic Bonding(2).pdf (PDF, 1.68 MB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000402126.