lec5 (PDF)
File information
Title: Antimicrobial-agent
Author: اØمد ÙŠØيى توÙيق
This PDF 1.5 document has been generated by Microsoft® Word 2016, and has been sent on pdf-archive.com on 16/11/2016 at 19:04, from IP address 5.1.x.x.
The current document download page has been viewed 427 times.
File size: 1.05 MB (10 pages).
Privacy: public file




File preview
ANTIMICROBIAL-AGENT
احمد يحيى توفيق
Definition
Drugs have been used for the treatment of infectious diseases since the 17th century (e.g.
quinine for malaria, emetine for amebiasis); however, chemotherapy as a science began in
the 1st decade of the 20th century with understanding of :
1. The principle of selective toxicity.
2.
The specific chemical relationships between microbial pathogens and drugs.
3.
The development of drug resistance.
4. The role of combined therapy.
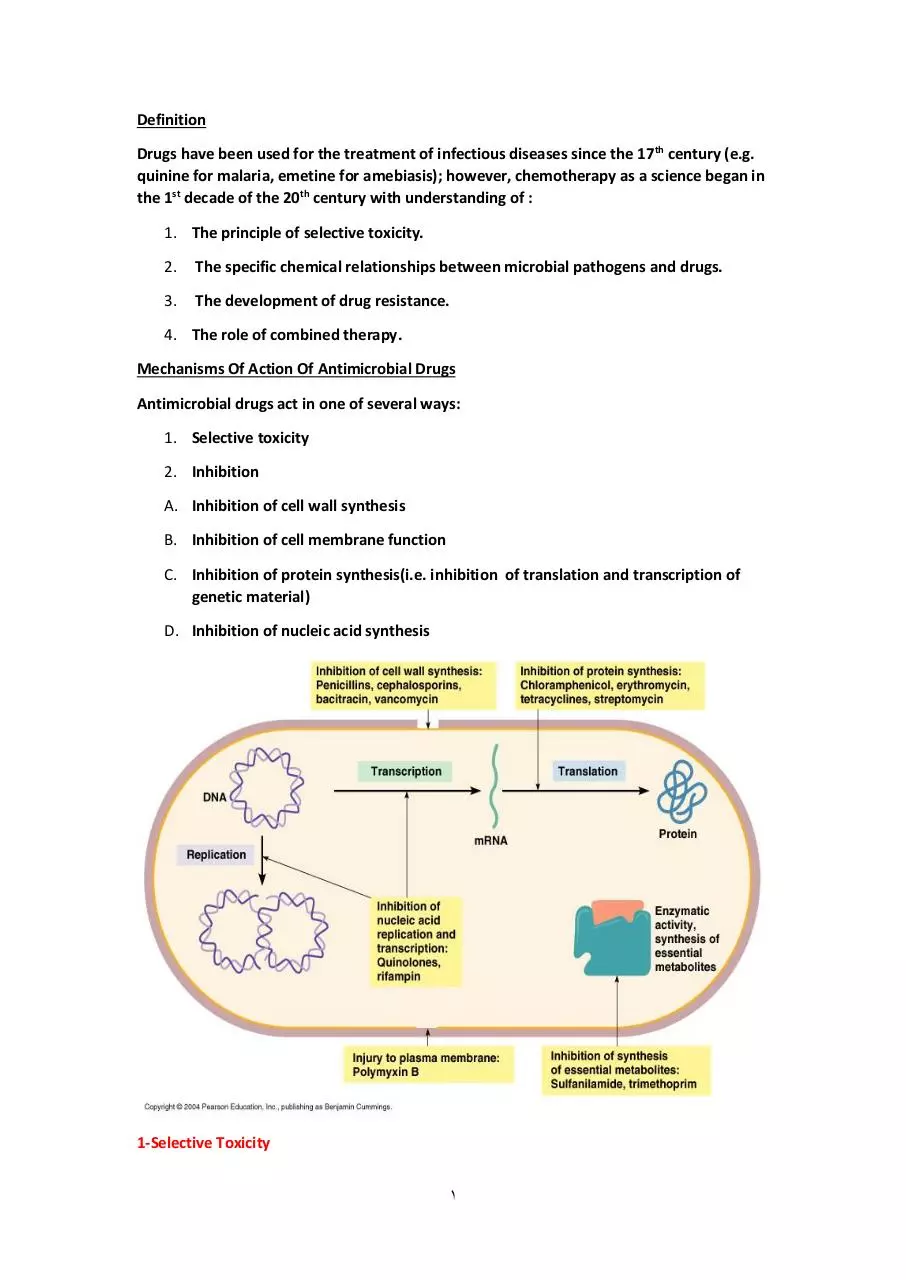
Mechanisms Of Action Of Antimicrobial Drugs
Antimicrobial drugs act in one of several ways:
1. Selective toxicity
2. Inhibition
A. Inhibition of cell wall synthesis
B. Inhibition of cell membrane function
C. Inhibition of protein synthesis(i.e. inhibition of translation and transcription of
genetic material)
D. Inhibition of nucleic acid synthesis
1-Selective Toxicity
1
An ideal antimicrobial agent exhibits selective toxicity, which means that the drug is
harmful to a pathogen without being harmful to the host.
Selective toxicity may be a function of:
A. A specific receptor required for drug attachment,
B.
It may depend on the inhibition of biochemical events essential to the pathogen
but not to the host.
2-inhibition
a-Inhibition Of Cell Wall Synthesis
Bacteria have a rigid outer layer. The cell wall maintains the shape and size of the
microorganism, which has a high internal osmotic pressure. Injury to the cell wall (eg. By
Lysozyme) or inhibition of its formation may lead to lysis of the cell.
In a hypertonic environment (eg. 20% sucrose), damage cell wall formation leads to
formation of spherical bacterial “Protoplasts” from gram-positive organism or
“Spheroplasts” from gram-negative organisms; these forms are limited by the fragile
cytoplasmic membrane.
when protoplasts or spheroplasts are placed in an environment of ordinary tonicity, they
take up fluid rapidly, swell and may explode
β -Lactam drugs
All β-lactam drugs are selective inhibitors of bacterial cell wall synthesis.
2
The initial step in drug action consists of binding of the drug to cell receptors (Penicillinbinding proteins; PBPs). There are 3 – 6 PBPs, some of which are transpeptidation enzyme
.
Different receptors have different affinities for a drug, and each may mediate a different
effect. For example, attachment of penicillin to one PBP may result in abnormal elongation
of the cell, whereas attachment to another PBP may lead to a defect in the periphery of
the cell wall, which resulting cell lysis.
The difference in susceptibility of gram-positive and gram-negative bacteria to various
penicillin's or cephalosporin's probably depends on:
1. structural differences in their cell wall(eg):
A.
amount of peptidoglycan
B.
presence of receptors
C. presence of lipids
D. Nature of cross-linking
E. Activity of autolytic anzymes
2. penetration, binding and activity of drugs.
Resistance to penicillin's may be determined by organisms production of penicillindestroying enzymes(β-lactamases). β-lactamases open the β-lactam ring of penicillins and
cephalosporins and abolish their antimicrobial activity. β-lactamases have been described
for many species gram-positive and gram-negative bacteria.
These inhibitors protect simultaneously present hydrolyzed penicillin's (eg. Ampicillin,
amoxicillin, and ticarcillin) from destruction.
Agents include
Amoxicillin-clavulanic Acid
3
Ampicillin- sulbactam
Ceftaroline- avibactam
Ceftazidime-avibactam
Piperacillin- tazobactam
Ticarcillin-clavulanic Acid
Penicillin
Antimicrobial Subclass
Agents include
Penicillin
penicillin
Aminopenicillin
Ampicillin
Amoxicillin
Ureidopenicillin
Azlocillin
Mezlocillin
Piperacillin
Carboxypenicillin
Carbenicillin
Ticarcillin
Penicillinase-stable penicillins
Cloxacillin
Dicloxacillin
Methicillin
Nafcillin
Oxacillin
Amidinopenicillin
Mecillinam
Cephalosporin
Antimicrobial Class:
Cephems (Parenteral)
Cephalosporines have been arranged into major group or generations:
1.First-Generation Cephalosporins:
4
Very active against gram-positive cocci – except enterococci and methicillin-resistant
staphylococci(MRSA), and moderately active against some gram-negative rods – primarily
E. coli, Proteus, and Klebsiella.
None of the 1st generation drugs penetrate the CNS and they are not drugs of choice for
any infection
a) Cephalothin
b) Cephapirin
c) Cefazolin
d) Cephradine
2. Second Generation Cephalosporins:
All are active against organisms covered by 1st generation drugs but have extended
coverage against gram-negative rods
a) Cefamandole
b) Cefuroxime (Parenteral)
c) Cefonicid
3. Third-Generations Cephalosporins:
Have decreased activity against gram-positve cocci, except for Strep. Pneumoniae,
enterococci but enhanced activity against gram-negative rods .
3rd generation drugs – except Cefoperazone is ability to reach the CNS
a) Cefotaxime
b) Ceftizoxime
c) Ceftriaxone
d) Ceftazidime
e) Cefoperazone
4. Fourth – Generation Cephalosporins:
Cefepime is the only 4th – generation cephalosporin. It has enhanced activity against
Enterobacter and Citrobacter speciesthat resistant to 3rd generation cephalosporins.
5. Cephalosporins with Anti-MRSA activity:
Ceftaroline
Ceftobiprole
Antimicrobial Class:
Cephems (Oral)
Subclass :
5
Cephalosporin
Cefaclor
Cefprozil
Cefadroxil
Ceftibuten
Cefdinir
Cefuroxime(Oral)
Cefditoren
Cephalexin
Cefetamet
Cephradine
Cefixime
Cefpodoxime
b-Inhibition of cell membrane function
The cytoplasm of all living cells is bounded by the cytoplasmic membrane, which serves as
I.
A selective permeability barrier
II.
Carries out active transport
III.
Control the internal composition of the cell
If the functional integrity of the cytoplasmic membrane is disrupted, macromolecules and
ions escape from the cell, and cell damage or death ensues.
The cytoplasmic membrane of bacteria and fungi has a structural different from that of
animals cells and can be more readily disrupted by certain agents. Consequently selective
chemotherapy is possible.
c-Inhibition of protein synthesis
It is established that erythromycins, lincomycins, tetracyclines, aminoglycosides, and
chloramphenicol can inhibit protein synthesis in bacteria.
Bacteria have 70S ribosome's, whereas mammalian cells have 80S ribosome's. The
subunits of each type of ribosome, their chemical composition, and their functional
specificities are sufficiently different to explain why antimicrobial drugs can inhibit protein
synthesis in bacterial ribosome's without having a major effect on mammalian ribosome's.
In normal microbial protein synthesis, the mRNA message is simultaneously “read” by
several ribosome's that are strung out along the mRNA strand. These are called
polysomes.
Aminoglycosides
The mode of action of streptomycin has been studied far more intensively than that of
other aminoglycosides, but all probably act similarly.
1st step is attachment of the aminoglycoside to a specific receptor protein (P12 in the case
of streptomycin) on the 30S subunit of the microbial ribosome.
6
2nd the aminoglycoside blocks the normal activity of the “initiation complex” of peptide
formation (mRNA + formyl methionine + tRNA).
3rd the mRNA message is misread on the “recognition region” of the ribosome;
consequently, the wrong amino acid is inserted into the peptide, resulting in a
nonfunctional protein.
4th aminoglycoside attachment results in the breakup of polysomes and their separation
into monosomes incapable of protein synthesis.
5th overall effect is usually an irreversible event-killing of the bacterium.
•
Chromosomal resistance of microbes to aminoglycosides, depends on lack of a
specific protein receptor on the 30S subunit of the ribosome.
•
Plasmid-dependent resistance to aminoglycosides depends on the production by
the microorganism of adenylylating, phosphorylating, or acetylating enzymes that
destroy the drugs.
A 3rd type of resistance consists of a “permeability defect” an outer membrane change that
reduces active transport of the aminoglycoside into the cell so that the drug cannot reach
the ribosome. Often this is plasmid-mediated.
Antimicrobial Class: Aminoglycosides
Agents include
Amikacin
Gentamicin
Kanamycin
Netilmicin
Plazomicin
Streptomycin
Tobramycin
7
d-Inhibition of Nucleic Acid Synthesis
For many microorganisms, P-aminobenzoic acid (PABA) is an essential metabolite. The
specific mode of action of PABA involves an adenosine triphosphate (ATP)-dependent
condensation of a pteridine with PABA to yield dihydropteroic acid, which is subsequently
converted to folic acid. PABA is involved in the synthesis of folic acid, an important
precursor to the synthesis of nucleic acids.
Sulfonamide are structural analogs of PABA and inhibit dihydropteroate synthesis.
Sulfonamides can enter into the reaction in place of PABA and compete for the active
center of the enzyme. As a result, nonfunctional analogs of folic acid are formed,
preventing further growth of the bacterial cell. The inhibiting action of sulfonamides on
bacterial growth can be counter-acted by excess of PABA in the environment (competitive
inhibition)
Antimicrobial Activity in vivo
Analysis of the activity of antimicrobial agents in vivo is much more complex than the
circumstances in vitro. The activity involves not only the drug and organism but also 3rd
factor, the host. Drug-pathogen and host-pathogen relationships.
Drug-pathogen relationships:
1. Environment
Varying environmental influences affect microorganism located in different tissues and
different part of body.
A. State of metabolic activity
Many organism exist at a low level of biosynthesis activity and depend on host metabolic
activity, so they insusceptible to drug action
B. Distribution of drug
Distribution unequally in tissue and fluid. Many drug do not reach the CNS, and
concentration in urine more in blood.
8
Download lec5
lec5.pdf (PDF, 1.05 MB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000508176.