2009 Sponsored Links Enforcement Past and Present (PDF)
File information
Author: nicodemo fiorentino
This PDF 1.6 document has been generated by Microsoft® Word 2016, and has been sent on pdf-archive.com on 27/07/2017 at 16:50, from IP address 47.22.x.x.
The current document download page has been viewed 403 times.
File size: 2.26 MB (18 pages).
Privacy: public file



File preview
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
OPDP’s 2009 Sponsored Links Enforcement Actions Against Fourteen
Manufacturers:
A Look at How Those with Products with a Boxed Warning Are Advertising on
Google Now
BACKGROUND
In April 2009, FDA’s Division of Drug Marketing, Advertising, and Communications (DDMAC) (now
Office of Prescription Drug Promotion) issued fourteen (14) enforcement letters against
pharmaceutical manufacturers for improper sponsored link promotion.
The sponsored links cited in these letters were considered to be misleading because they made
representations and/or suggestions about the efficacy of the products, but failed to communicate
any risk information associated with the use of these drugs.
In addition, the sponsored links inadequately communicated the drugs’ indications.
Some sponsored links also failed to use the required established name. Thus, DDMAC believed
the sponsored links misbranded the drugs in violation of the Federal Food, Drug, and Cosmetic Act
(the Act) and FDA implementing regulations.
DDMAC cited the following authorities: 21 U.S.C. 352(a) & (n), 321(n); 21 CFR 201.10(g)(1),
202.1(b)(1), (e)(3)(i), (ii) & (e)(6)(i).
DDMAC noted that the omission of risk information was particularly concerning for those products that
have Boxed Warnings. In addition, DDMAC stated “[f]or promotional materials to be truthful and nonmisleading, they must contain risk information in each part as necessary to qualify any claims made
about the drug.”
Click here to see the enforcement letters and promotional materials.
PRODUCTS WITH BOXED WARNINGS
1. Cymbalta
2. Evista
3. Pegasys
4. Xeloda
5. Avastin
6. Rituxan
7. Herceptin
8. Xolair
9. Diovan
10. Exforge
11. Celebrex
12. Fentora
13. Lexapro
14. Avandia
15. Avandamet
16. Avanaryl
17. Tykerb
18. Yaz
19. Tysabri
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
1
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
CURRENT SPONSORED LINK PROMOTION: IS IT TIME FOR OPDP TO FLEX ITS MUSCLES
ONCE MORE?
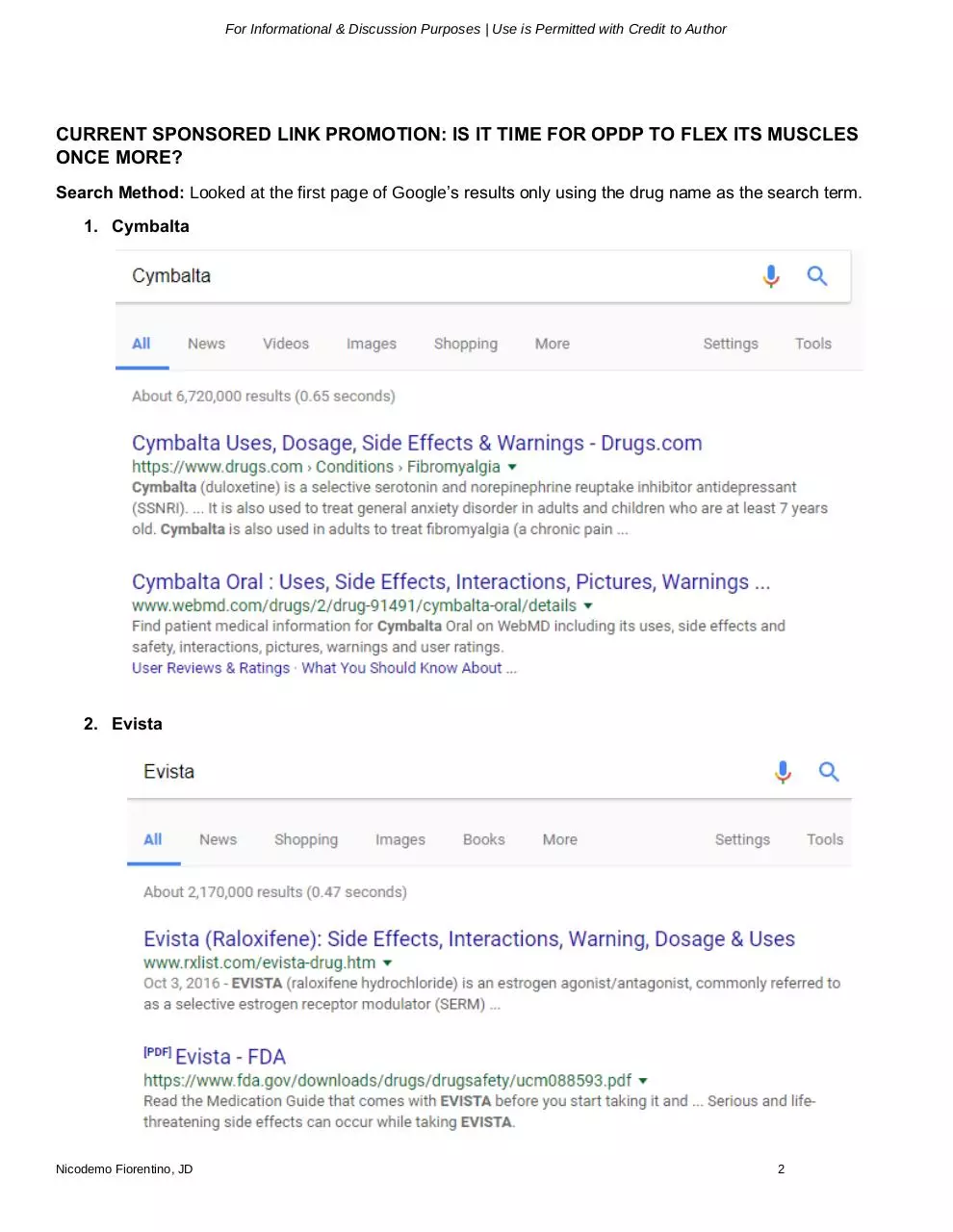
Search Method: Looked at the first page of Google’s results only using the drug name as the search term.
1. Cymbalta
2. Evista
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
2
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
3. Pegasys
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
3
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
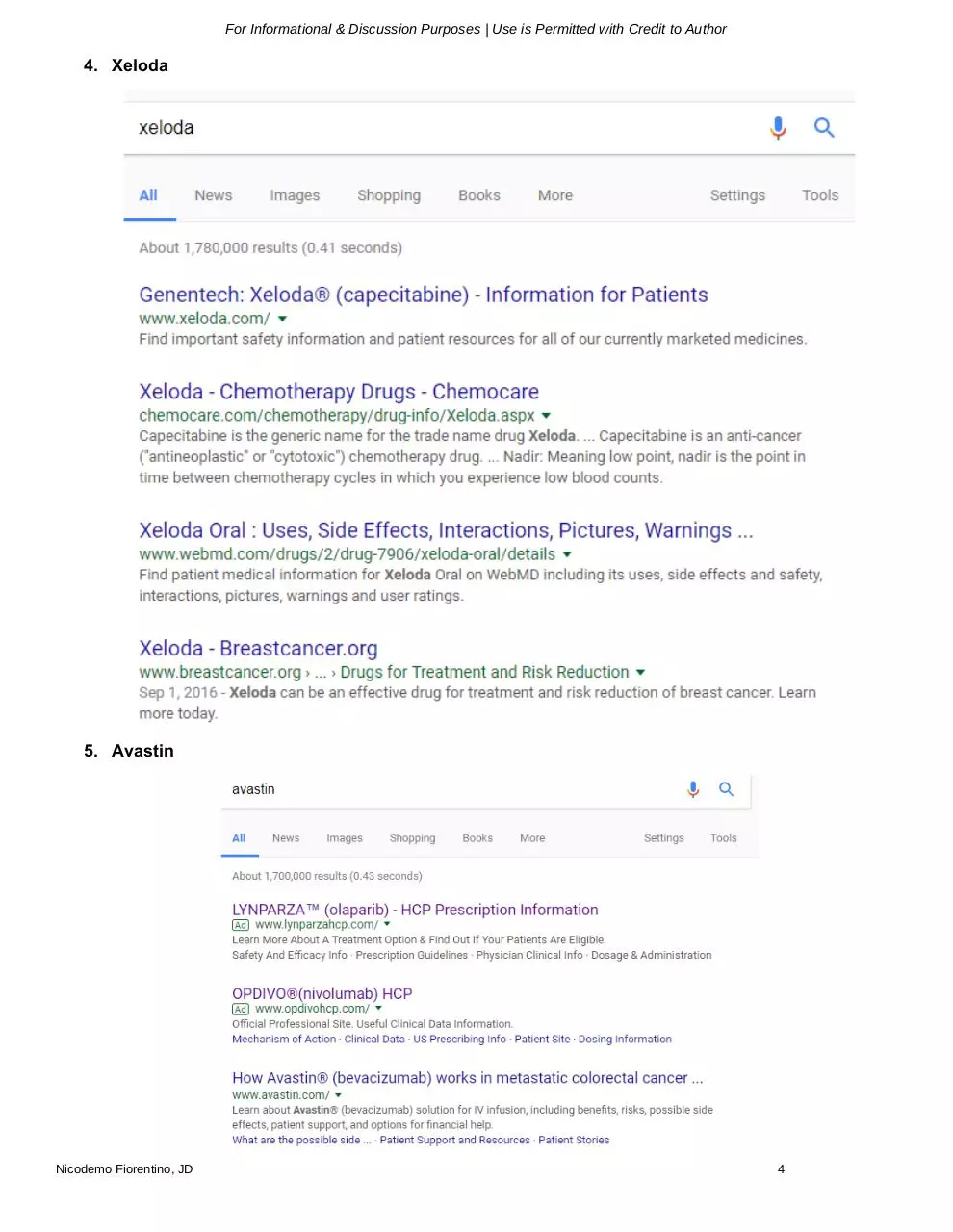
4. Xeloda
5. Avastin
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
4
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
6. Rituxan
7. Herceptin
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
5
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
8. Xolair
9. Diovan
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
6
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
10. Exforge
11. Celebrex
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
7
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
12. Fentora
13. Lexapro
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
8
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
14. Avandia
15. Avandamet
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
9
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
16. Avandaryl
17. Tykerb
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
10
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
18. Yaz
19. Tysabri
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
11
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
2009 ENFORCEMENT LETTERS AND VIOLATIVE PROMOTIONAL MATERIAL (snapshot of
violative piece included; click on “Promotional Material” to see full violative piece)
1. Biogen - BLA 125104 Tysabri (natalizumab) injection for intravenous use
FDA/DDMAC Letter
Promotional Material
2.
Sanofi - NDA 20-839 Plavix (clopidogrel bisulfate) Tablets
FDA/DDMAC Letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
12
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
3. Bayer- NDA 21-400 LEVITRA (vardenafil HCl) Tablets; NDA 21-676, 21-873, 22-045
YAZ (drospirenone and ethinyl estradiol) Tablets; NDA 21-225 Mirena (levonorgestrelreleasing intrauterine system)
FDA/DDMAC letter
Promotional Material
4. GSK - NDA 21-071 Avandia (rosiglitazone maleate) Tablets; NDA 21-410 Avandamet
(rosiglitazone maleate and metformin hydrochloride) Tablets; NDA 21-700 Avandaryl
(rosiglitazone maleate and glimepiride) Tablets; NDA 21-319 Avodart (dutasteride) Soft
Gelatin Tablets; NDA 22-012 Coreg CR (carvedilol phosphate) Extended-release
Capsules; NDA 22-059 Tykerb (lapatinib) Tablets
FDA/DDMAC Letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
13
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
5. Forest - NDA 21-742 BYSTOLIC (nebivolol) Tablets; NDA 21-431 CAMPRAL
(acamprosate calcium) Delayed-Release Tablets; NDA 21-323, 21-365, 21-440 Lexapro
(escitalopram) Tablets/ Oral Solution; NDA 21-487, 21-627 Namenda (memantine)
Tablets/Oral Solution
FDA/DDMAC letter
Promotional Material
6. Cephalon - NDA 21-947 FENTORA (fentanyl buccal tablet); NDA #22-249 TREANDA
(bendamustine hydrochloride) for Injection, for intravenous infusion
FDA/DDMAC Letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
14
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
7. J&J - NDA 21-976 PREZISTA (darunavir) Tablet
FDA/DDMAC letter
Promotional Material
8. Pfizer - NDA 20-753 AROMASIN (exemestane) tablets; NDA 21-540 CADUET
(amlodipine besylate/atorvastatin calcium) Tablets; NDA 21-928 CHANTIX®
(varenicline) Tablets; NDA 21-228 Detrol LA (tolterodine tartrate) extended release
capsules; NDA 21-446, 21-723, 21-724 LYRICA (pregabalin) Capsules; NDA 20-998,
21-156 CELEBREX (celecoxib) capsules
FDA/DDMAC letter
Promotional Material
9. Novartis - NDA 20-726 Femara (letrozole tablets); NDA 21-283 Diovan (valsartan)
Tablets; NDA 21-990 Exforge (amlodipine and valsartan) Tablets; NDA 21-882 EXJADE
(deferasirox) Tablets for Oral Suspension; NDA 21-588 GLEEVEC (imatinib mesylate)
Tablets for Oral Use
FDA/DDMAC letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
15
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
10. Genentech - BLA 125085 Avastin (Bevacizumab); BLA 125156 LUCENTIS
(ranibizumab injection); BLA 103705, 103737 RITUXAN (rituximab); BLA 103976 Xolair
(Omalizumab) For Subcutaneous Use; BLA 103792 HERCEPTIN (trastuzumab); BLA
103532 Pulmozyme (dornase alfa) Inhalation Solution
FDA/DDMAC letter
Promotional Material
11. BI - NDA 21-395 Spiriva HandiHaler (tiotropium bromide inhalation powder); NDA 20579 Flomax (tamsulosin hydrochloride) Capsules; NDA 20-667 Mirapex (pramipexole
dihydrochloride) Tablets
FDA/DDMAC letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
16
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
12. Merck - NDA 21-995 JANUVIA (sitagliptin) Tablets; NDA 20-788 PROPECIA
(finasteride) Tablets; NDA 20-829, 20-830, 21-409 SINGULAIR (montelukast sodium)
granule; tablet, chewable; tablet, film coated; NDA 21-549 EMEND (aprepitant)
Capsules
FDA/DDMAC letter
Promotional Material
13. Hoffman-LaRoche - NDA 21-455, 21-858 BONIVA (ibandronate sodium) Tablets; BLA
103964 PEGASYS (peginterferon alfa-2a) for Injection; NDA 20-896 XELODA
(capecitabine) Tablets
FDA/DDMAC letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
17
For Informational & Discussion Purposes | Use is Permitted with Credit to Author
14. Lilly - NDA 21-427, 21-733, 22-148 Cymbalta (duloxetine hydrochloride) DelayedRelease Capsules for Oral Use; NDA 20-815, 22-042 EVISTA (raloxifene hydrochloride)
Tablets for Oral Use; NDA 20-509 Gemzar (gemcitabine HCl) for Injection
FDA/DDMAC letter
Promotional Material
nicodemo fiorendtino, jd
Nicodemo Fiorentino, JD
18
Download 2009 Sponsored Links Enforcement Past and Present
2009 Sponsored Links Enforcement_Past and Present.pdf (PDF, 2.26 MB)
Download PDF
Share this file on social networks
Link to this page
Permanent link
Use the permanent link to the download page to share your document on Facebook, Twitter, LinkedIn, or directly with a contact by e-Mail, Messenger, Whatsapp, Line..
Short link
Use the short link to share your document on Twitter or by text message (SMS)
HTML Code
Copy the following HTML code to share your document on a Website or Blog
QR Code to this page

This file has been shared publicly by a user of PDF Archive.
Document ID: 0000629529.